Regulation and 3 dimensional culture of tertiary follicle growth
Article information
Abstract
It has been revealed that multiple cohorts of tertiary follicles develop during some animal estrous cycle and the human menstrual cycle. To reach developmental competence, oocytes need the support of somatic cells. During embryogenesis, the primordial germ cells appear, travel to the gonadal rudiments, and form follicles. The female germ cells develop within the somatic cells of the ovary, granulosa cells, and theca cells. How the oocyte and follicle cells support each other has been seriously studied. The latest technologies in genes and proteins and genetic engineering have allowed us to collect a great deal of information about folliculogenesis. For example, a few web pages (http://www.ncbi.nlm.nih.gov; http://mrg.genetics.washington.edu) provide access to databases of genomes, sequences of transcriptomes, and various tools for analyzing and discovering genes important in ovarian development. Formation of the antrum (tertiary follicle) is the final phase of folliculogenesis and the transition from intraovarian to extraovian regulation. This final step coordinates with the hypothalamic-pituitary-ovarian axis. On the other hand, currently, follicle physiology is under intense investigation, as little is known about how to overcome women's ovarian problems or how to develop competent oocytes from in vitro follicle culture or transplantation. In this review, some of the known roles of hormones and some of the genes involved in tertiary follicle growth and the general characteristics of tertiary follicles are summarized. In addition, in vitro culture of tertiary follicles is also discussed as a study model and an assisted reproductive technology model.
Introduction
Folliculogenesis is the process by which the female germ cell develops within the somatic cells of the ovary and matures into a fertilizable oocyte (Figure 1A) [1]. Newborn mouse ovaries are densely packed with oocytes called naked oocytes (germ cell cysts, clusters, nests, or syncytia) [2,3], most of which are present in clusters with no evidence of surrounding granulosa cells [2]. The oocytes in the cluster are connected by intercellular bridges (Figure 1B). Most of the oocytes enter meiosis during embryonic life, and at birth, some oocytes are in the transitory stages of prophase I. Folliculogenesis is initiated by coupling between the primary oocyte and ovarian stromal cells. One to 2 days after birth, a number of oocytes are surrounded by flat squamous pre-granulosa cells (primordial follicle) (Figure 1C). By postnatal day 3, the pre-granulosa cells differentiate to granulosa cells and the oocyte reaches growth beyond 20 µm. At postnatal day 7, most of the germ cell cysts have disappeared and primordial follicles are the most abundant follicular type, but primary and secondary follicles are present in the medullary region (Figure 1D, E). By postnatal day 21, early antral (early tertiary) follicles are observed and the size of oocytes reaches its final diameter. In contrast, in humans, primordial follicles are formed during gestation. Primordial follicles are observed after 20 weeks of gestation, and preantral (secondary) and antral (tertiary) follicles are observed from 26 weeks onwards (Figure 1F, G). In newborn human females, approximately 400,000 follicles remain. Follicular maturation and atresia occur prenatally and throughout childhood [4,5]. During folliculogenesis, follicles have to be recruited and selected to participate in ovulation. "Recruitment" of follicles can have several meanings, but here it refers to the emergence of a cohort of medium-size follicles. "Selection" refers to the preferential growth of the dominant follicle from the cohort of recruited tertiary follicles.
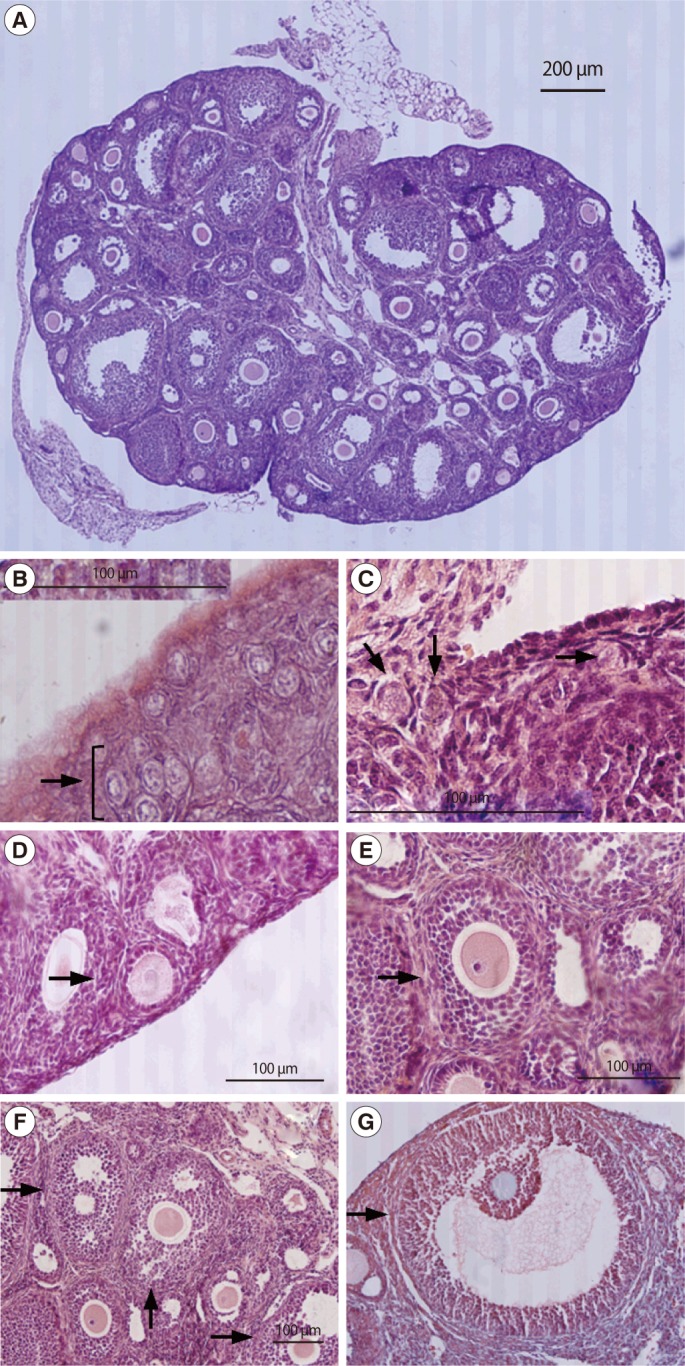
Photomicroscopy of mouse follicles for follicle classification. Sections of whole ovary (A, ×40), germ cell cyst (B, ×400), primordial follicle (C, ×400), primary follicle (D, ×400), secondary follicle (E, ×400), early tertiary follicle (F, ×200), and late tertiary follicle (G, ×200) were stained with H-E and the arrows are indicated each specific stage follicles.
Multiply recruited follicles develop during the estrous cycle of several animal species and the menstrual cycle of humans. Recruited follicle develops an antrum between the granulosa cells [6]. Tertiary follicle formation is associated with continued proliferation of granulosa and theca cells, further increased thecal vascularization, and further oocyte enlargement. By the transition from secondary to tertiary follicles, the ovary can clearly works as an endocrine organ. Finally, the grown tertiary follicles release matured oocytes. Development and differentiation of tertiary stage follicles is important in selection of dominant follicles, choosing competent oocytes, preserving fertility, and helping fertilization. These processes are under the control of the hypothalamic-pituitary-ovary axis and the local regulation including hormones and cytokines. Using knockout mice, the critical genes that are important in tertiary follicle growth and functional regulation have now been identified. However, they are very complex, so they are not fully understood and so cannot yet be applied to assisted reproduction. For example, the mechanisms of the explosive growth of tertiary follicles have not been fully elucidated. In vitro culture of follicles can help in exploring the role of various candidate genes and chemicals in folliculogenesis. Using an in vitro culture system, we can now obtain competent oocytes from follicles or oocyte-cumulus complexes involved in producing a new generation, although the yield is low. Therefore, much more efficient in vitro culture technology is needed to improve the quality of oocytes and our understanding of their mechanisms.
General characteristics of tertiary follicles
Recruited late secondary follicles cannot form tertiary follicles without sufficient gonadotropin stimulation. Gonadotropins trigger the antral formation, the expression of related genes, and the selection of the dominant follicles. In the early tertiary stage follicles, the oocyte grows to the final diameter of approximately 70 µm and is surrounded by multiple layers of granulosa cells that contain scattered areas of interstitial fluid (Figure 1F). These scattered areas of fluid coalesce to form the antral cavity (Figure 1G) [7].
Gonadotropins trigger the expression of downstream genes and the downstream genes regulate the growth and differentiation of follicle cells. FSH is survival factor of early tertiary follicles; without FSH, these follicles undergo apoptosis [8]. In the dominant follicles, LH stimulates theca cell androgen production, while FSH stimulates granulosa cell proliferation, aromatization of androgens to estrogen, and LH receptor expression [9]. Inhibin and activin, secreted by granulosa cells are involved in the feedback regulation of FSH secretion [10]. Thereby, tertiary stage follicles participate actively in the hypothalamic-pituitary-ovarian axis.
The structural connection between oocyte and granulosa cells is critical for antrum formation and oocyte competency. An oocyte builds its own microenvironment in the follicle and controls the differentiation of the follicle cells and its own. Microtubule transzonal projections (TZPs) mediate the structural formation of TZPs between the oocytes and granulosa cells [11]. After selection by the complicated web of interactions among the oocyte, granulosa cells, and theca cells, an FSH and LH surge triggers the release of oocytes.
The growth of tertiary follicles has a well-characterized pattern of growth and development. Growth of tertiary follicles includes expansion of the antrum and the increase in the number of follicle cells. It is known that granulosa and theca cells continue to undergo mitosis concomitant with an increase in antrum volume. On the other hand, as follicle enlarge, the granulosa cells and theca cells progressively stop dividing to express differentiated functions. Differentiation of theca and granulosa cells is under the control of FSH, LH, prolactin, and local regulators, which can be paracrine or autocrine. This is observed when the follicle diameter reaches a specific size, such as 6 mm in humans. The increase in the ability of theca cells to produce androgen coincides with the increase in the ability of granulosa cells to aromatize them into estrogen. Angiogenic factors are also important in tertiary follicular growth, particularly the selective growth of oocytes and follicles. Epidermal growth factor and GAGs are included in angiogenic activity [12].
During the tertiary follicle stage, to support the fertility of the ovulated oocyte, the oocyte is protected in various ways during follicular extrusion and is assisted in sperm binding and preparation for fertilization. Hyaluronic acid (HA), prostaglandin E2, pentraxin 3, and tumor necrosis factor alpha induced protein 6 (Tnfaip6) are involved in these processes [13]. Tnfaip6-/- mice lack hyaluronan and heavy chain inter-α-trypsin inhibitor family complexes [14]. In the ovulated oocytes of Ptx3 knockout mice, the cumulus cells are missing [13].
Role of hormones in the growth of tertiary follicles
The growth of tertiary follicles is controlled both at the system and local levels. In system level control, the hypothalamus-pituitary-ovarian axis is known to be the main regulator. FSH is the main regulator of recruiting the secondary follicles to grow to tertiary follicles. The actions of FSH are modulated at the system level by tissue receptivity (number and affinity of receptors) and by local regulators (steroids and proteins) that increase or decrease the follicular response to gonadotropins. FSH triggers gene expression which is involved in antral formation, in preparing for ovulation, and in oocyte competency [15]. It is cleared from FSH beta (Fshb) knockout mice. In Fshb knockout mice, follicles arrest at the late secondary follicle (preantral) stages [16,17]. Complete restoration of folliculogenesis in Fshb null mice is accomplished by the expression of FSH [18]. FSH receptor (Fshr) null mice have changes in oocyte-granulosa cell communication [19]. In these mice, reduced oocyte growth is observed and the protein levels of KIT, epithelial growth factor kit ligand (KITL), and bone morphogenic protein 15 (BMP-15) are decreased [19]. Serum/glucocorticoid inducible kinase, Cypll1, and aromatase (CYP19) are down-regulated significantly in the ovaries of Fshr null mice [20]. Arresting of tertiary follicle development in insulin-like growth factor 1 (Igf1) null female mice is caused by a low expression levels of FSHR [21,22].
In women with mutant in Fshb, primary amenorrhea, absent breast development, low FSH, high LH, and undetectable estrogen are detected. The observed phenotype in women with the Fshb mutations mimics well the mouse Fshb gene mutation [23]. In the case of mutations in the Fsh gene, follicles develop to the secondary stage as in Fshr null mice [24].
LH is critically involved in tertiary follicle growth and maturation. Basal levels of LH are important for recruiting of secondary follicles to grow to become tertiary follicles [25]. LH stimulates androgen production in theca cells. These androgens are transformed into estrogen through the action of the aromatase system [26]. LH beta (Lhb) null female mice are infertile. Primary and secondary follicles with intact theca cell layers develop but healthy large tertiary follicles are absent. However, in LH receptor (Lhcgr) knockouts, late secondary and tertiary follicles are present. As compared with Fshr knockouts, Lhcgr knockouts have more advanced follicular structures [27,28].
Estrogen is synthesized in the granulosa cells of developing follicles. It stimulates granulosa cell growth, increases the synthesis of IGF1, maintains FSHR, induces LH receptor expression, augments aromatase activity and subsequent estrogen production, and attenuates granulosa cell apoptosis. Estrogen stimulates the transition from the secondary to tertiary stage of folliculogenesis and alters the ratio of follicular parenchyma to ovarian stroma in favor of stroma [29]. Estrogen receptor beta (Esr2) is expressed at a higher level in the granulosa cells of tertiary follicles than in estrogen receptor alpha (Esr1) is expressed in theca cells and interstitial cells [30, 31]. Using Esr1, Esr2, and aromatase knockout mouse models, estrogen appears to play an important role in the feedback regulation of ovulation and continued follicle growth but does not interfere with oocyte growth [31-33]. A woman who has a mutation in the cytochrome P450 (Cyp19a1, P450 aromatase) will have primary amenorrhea and cystic ovaries [34,35].
Growth hormone receptor (Ghr) RNA is localized in the granulosa cells of tertiary follicles [36]. IGF1 does not restore the ovulation rate in GH receptor knockout mice. In Ghr knockouts, the number of apoptotic follicles is increased [37].
Leptin is expressed in oocytes, granulosa cells, and theca cells. It increases the meiotic resumption in fully grown follicle-enclosed oocytes [38]. Leptin is highly expressed in the theca cells of antral follicles. Although leptin is expressed in the oocytes and follicle cells, it is thought that non-gonadal leptin can restore fertility [39]. Fertility of ob/ob mice can be restored by replacement of leptin or gonadotropins [39]. Leptin can suppress ovarian steroid synthesis and IGF1 synergistic effects with FSH on estrogen production in granulosa cells and also inhibits aromatase activity [40,41]. It has also been suggested that leptin-induced angiogenesis may be important in folliculogenesis [42].
Activins (isoforms: A [βA homodimer], AB [βA βB heterodimer], and B [βB homodimer]) and inhibins (isoforms: A [α and βA heterodimer] and B [α and βB heterodimer]) are synthesized in ovarian follicles, the pituitary gland, placenta, and other organs. Inhibin and activin have almost directly opposite biological effects. In the ovaries, activin is synthesized in the granulosa cells of tertiary follicles and is involved in the negative feedback loop of FSH. Activin supports FSH secretion in the pituitary and is involved in the cyclic recruitment of follicles. Activin A inhibits spontaneous luteinization in mature tertiary follicles through the inhibition of LH-induced production of progesterone and oxytocin in granulosa cells [43]. It also attenuates LH-dependent androgen production in theca cells [44]. The major activin signaling receptor is activin receptor type IIa (Acvr2a). Acvr2a null mice have a few tertiary follicles and follicular atresia and rare corpora lutea [45]. The effect of activin is under the control of follistatin.
In the ovaries, inhibin is synthesized in the granulosa cells of tertiary follicles and is involved in the negative feedback loop of FSH. It is suggested that inhibin is needed in late stage folliculogenesis in mice. Inhibin α (Inha) null mice develop ovarian tumors [10]. If the ovaries from the inhibin α null mice are transplanted to an immunocompatible host prior to gross tumor development, the ovaries have few follicles beyond the preantral stage. Furthermore, from the superovulation induced in these mice, the ovulated number of oocytes is much lower compared to controls [46]. Inhibin A synthesis increases in the granulosa cells of selected tertiary follicles and enhances LH-induced androgen production [47]. In this way, granulosa cells are able to maintain a sufficient supply of thecal androgen required for their greatly increased estrogen synthesis during the pre-ovulatory phase [48].
Local levels of inhibin and activin change according to the stage of folliculogenesis. It has been proposed that an orderly transition from an inhibin B/activin follicular environment to an inhibin A/follistatin environment is critical for dominant follicle development in women [49]. As follicles reach the size at which the FSH-dependent follicle selection mechanism operates, these follicles produce more activin relative to inhibin, while larger selected follicles secrete proportionally more inhibin [50,51]. A changing intrafollicular balance between inhibin and activin contributes to granulosa cell proliferation and differentiation, androgen synthesis in theca cells, and oocyte support and development. Interestingly, inhibin A, or its free α-subunit has a negative effect on both oocyte maturation and developmental competence. In contrast, activin A accelerated oocyte maturation [52,53] and developmental competence [54].
Antimullerian hormone (AMH) is synthesized in the granulosa cells of secondary and early tertiary follicles. The levels of intrafollicular AMH gradually decrease during tertiary follicle growth until selection time. Its expression is under the control of FSH and estrogen. AMH reduces the FSH responsiveness of small tertiary follicles [55].
Important genes in the growth of tertiary follicles
It is well known that growth factors originate from follicle cells and oocytes play an important role in control of tertiary follicles such as follicle cell proliferation, apoptosis, release of hormones, and response to upstream hormonal responses [56]. A few kinds of growth factors in null mice have shown follicles arrested at the secondary or tertiary stages. Igf1 and its receptor is expressed in granulosa cells of growing follicles and increases during antral follicle growth. Igf1 knockout mice do not contain tertiary follicles and cannot ovulate [21,57]. Forkhead/winged helix transcription factor (Foxc1) is one of the transforming growth factor beta1 (TGF-β1)-responsive gene in the ovarian cell line [58]. The transplanted ovaries of Foxc1 null mice have no follicles developed beyond the early tertiary follicle, because theca and granulosa cell layers are disrupted [59]. In cyclin D2 null female mice, arresting of tertiary follicle development is observed at the same secondary follicle stages as Fshb null ovaries [16]. In connexin 43 (Gja1) null mice, late tertiary follicles are not observed [60-62].
Some factors are involved in the interconnection between hormones and local communicators during growth of tertiary follicles. BMPs that are expressed in the granulosa cells of tertiary follicles promote the proliferation and FSH-dependent function of granulosa cells. Those include BMP-2, -5, and -6. These facilitate follicle survival and the prevention of premature luteinization and/or atresia [63,64]. BMP-2 promotes estrogen and inhibin secretion from granulosa cells [65]. In addition these BMPs have a paracrine role in theca cells and attenuate LH-dependent androgen production in small- to medium-sized tertiary follicles. BMP-5 reduces FSH-stimulated progesterone production. BMP-6 derived from granulosa cells down-regulates adenylate cyclase activity and attenuates FSH action on granulosa cells [66]. At the time of dominant follicle selection, the dramatic loss of BMP-6 mRNA levels has been noted [67]. BMP-6 inhibits gonadotropin-stimulated progesterone secretion but has no effect on P450arom expression of estrogen secretion [66]. It enhances basal and IGF-stimulated estrogen, inhibin A, activin A, follistatin secretion, and cell number [66].
BMPs that are expressed in the theca cells of tertiary follicles promote granulosa cell proliferation and improve follicle survival and prevention of premature luteinization and/or atresia. They include BMP-2, -3b, BMP-4, and -7 [48,64,66]. BMP-2 promotes estrogen and inhibin secretion from granulosa cells [65]. BMP-4 attenuates FSH-stimulated progesterone concomitant with a decrease in steroidogenic acute regulatory (StAR) and P450scc, but enhances FSH-stimulated estrogen, inhibin A, activin A, and follistatin secretion, without affecting cell proliferation [66,68,69]. BMP-4 also suppresses basal and LH-induced androgen secretion and enhanced cell proliferation in theca cells [66]. BMP-7 performs autocrine and paracrine actions in tertiary follicles and enhances theca cell proliferation [66]. In granulosa cells, BMP-7 attenuates FSH-stimulated progesterone and enhances FSH-stimulated estrogen, inhibin A, activin A, and follistatin secretion, without affecting cell proliferation [66,68]. BMP-7 suppresses basal and LH-induced androgen secretion.
The biological function of BMPs is antagonized by BMP-binding proteins such as follistatin, noggin, chordin, gremlin, NBL1, and BMP and activin membrane-bound inhibitor (BAMBI). These BMP-binding proteins express in tertiary follicles and block BMP action. Follistatin can bind BMP-4, -6, and -7 and inhibit their effects on granulosa cells although evidently not on theca cells [66]. It can prevent the inhibitory actions of BMP-15 on FSH receptor expression [66]. Noggin neutralizes BMP-2 and BMP-4 action on granulosa cells [70]. Gremlin suppresses BMP-4 signaling in granulosa cells [71]. The NBL1 gradient is established by oocyte-derived growth/differentiation factor 9 (GDF-9), and its gradient serves as an antagonist barrier that spatio-temporally modulates the actions of theca-derived BMP-4 and granulosa/theca derived BMP-2 during folliculogenesis.
TGF-β is expressed in both theca and granulosa cells during tertiary follicle development [72]. TGF-β receptor is distributed ubiquitously in most cell types. TGF-β stimulates FSH receptor expression [73], and amplifies FSH-induced aromatase activity, inhibin production, progesterone production, and LH receptor induction [74].
A few genes are known to be involved in the control of steroid hormone biosynthesis during the growth of tertiary follicles. TGF-β suppresses theca P450c17 expression and androgen production [75]. Hepatocyte growth factor (HGF) is derived mainly from theca and interstitial cells in tertiary follicles. HGF mediates steroidogenesis and suppresses the apoptosis of tertiary follicles. It induces granulosa cell proliferation [76].
Proliferation or apoptosis regulating genes have also been identified during tertiary follicle growth. Cyclin D2 promotes granulosa cell proliferation [22,77,78]. Extracellular-regulated protein kinases 1 and 2 mediate a decrease in granulosa cell proliferation [79]. Hoxa7 (one of the homeobox genes) is expressed in granulosa cells and is stimulated by GDF-9. It regulates granulosa cell proliferation [80]. Tumor necrosis factor-alpha (TNFα) is produced by cumulus cells and is involved in preventing fragmentation of oocytes [81]. Nodal is involved in promoting follicular atresia [82]. NAIP is expressed in granulosa cells and protects ovarian follicular cells from apoptosis [83].
Keratinocyte growth factor (KGF) (i.e., fibroblast growth factor-7) and the epithelial growth factor KITL interact to coordinate the growth of later-stage tertiary follicles [84]. A local feedback loop is built between theca cells and granulosa cells by these ligands. Theca cell-derived KGF and HGF stimulate granulosa cell-derived KITL expression, and KITL, in turn, can stimulate theca cell-derived KGF and HGF expression. In addition, FSH directly stimulates KITL expression and LH stimulates KGF and HGF expression in theca cells [85].
Control of tertiary follicle growth by oocytes
It has been found that oocytes control the rate of follicular development [86]. When oocytes extracted from secondary follicles were reaggregated with somatic cells from newborns' ovaries, and transplanted into the renal capsules of bilaterally ovariectomized host females, tertiary follicles were visible in 9 days. As a control, primordial oocytes were rearranged with the same somatic cells and after 9 days, only secondary follicles were observed without tertiary follicles [86]. Within tertiary follicles, the oocytes continue to influence the behavior of the granulosa cells via the production of specific oocyte-secreted factors, hence regulating its own microenvironment [87,88]. As mentioned regarding the cellular communication between the oocyte and follicle cells, TZPs connect between oocytes and granulosa cells.
At least 5 growth factors are synthesized in the oocytes. These include GDF-9, BMP-6, BMP-15, FGF-8, and TGF-β2 [89]. GDF-9 is known as an oocyte-specific factor and modulator of TZP formation and controller of folliculogenesis. GDF-9 is essential in antral follicle growth [17,90]. Granulosa cells of tertiary follicles highly express some genes such as hyaluronan synthase (Has) Ptx3, Tnfaip6, and gremlin, by the treatment of GDF-9 [12,71]. GDF-9 inhibits urokinase plasminogen activator (Plau), and LH receptor mRNA. Gdf9 null mice clearly demonstrate the role of oocytes in granulosa cell proliferation and follicular progression [91]. GDF-9 promotes granulosa cell proliferation and provides premature luteinization and/or atresia. GDF-9 exerts its effect via regulation of gonadotropin action [92]. It suppresses FSH-stimulated progesterone and E2 production through suppression of P450arom activity [92,93] and attenuates FSH-induced LH receptor formation. GDF-9 also regulates cumulus expansion and expression of several key granulosa cell-specific genes such as Has2, cyclooxygenase 2 (Ptgs2), and Star protein mRNA synthesis. It also can induce progesterone production without FSH. However, it suppresses Plau and Lhcgr mRNA synthesis [94]. It has been shown that GDF-9 works with BMPs to maintain cell proliferation and provide premature luteinization and/or atresia.
BMP-6 and -15 derived from oocytes promote granulosa cell proliferation and improve follicle survival and prevention of premature luteinization and/or atresia [95]. BMP-6 expressed by oocytes down-regulates adenylate cyclase activity and attenuates FSH action on granulosa cells [63]. At the time of dominant follicle selection, a dramatic loss of Bmp-6 mRNA levels has been noted [89]. BMP-6 inhibits gonadotropin-stimulated progesterone secretion but has no effect on P450arom expression of estrogen secretion [63,96]. It enhances basal and IGF-stimulated estrogen, inhibin A, activin A, and follistatin secretion and cell number [97]. BMP-15 is required for follicle development to the ovulatory stage and is required for modulation of TZP formation [17]. BMP-15 attenuates FHS action on rat granulosa cells through suppression of FSH receptor expression [98] and inhibits gonadotropin-stimulated progesterone secretion [63,69]. In humans and sheep, BMP-15 enhances the proliferation of granulosa cells [99]. In the granulosa cells of early tertiary follicles, BMP-15 binds with the highest affinity to BMP receptor type-1B (BMPR1B), resulting in SMAD1/5/8 phosphorylation [100]. BMPR1B is expressed in oocytes and granulosa cells of tertiary follicles in mice [101].
Intermedin (IMD)/adrenomedullin 2 (ADM2) is synthesized in oocytes and is known to be a regulator of cell-cell communication in cumulus-oocyte-complexes. It suppresses cumulus cell apoptosis [102]. Communication between oocytes and granulosa cells and between follicle cells is pivotal in folliculogenesis. It is well known that the structural protein connexin is important in maintaining communication between oocytes and cumulus cells. Connexin 37 gap junctions develop between oocytes and granulosa cells. Connexin 37 is involved in the transition from secondary to tertiary follicle development [61]. Connexin 43 (Gja1) gap junctions also connect granulosa cells and oocytes. Connexin 43 null granulosa cells are unable to proliferate. Connexin 43 null mice contain developing preantral follicles, but late tertiary follicles are not observed and produce incompetent oocytes [60-62]. Oocytes recovered from Gja1 mutants could not be fertilized [103]. Connexin 43 expression is under the control of FSH and LH. FSH causes induction of connexin 43 synthesis but a preovulatory surge of LH is followed by a drop in the level of connexin 43 [104-106]. Its expression regulation by gonadotropins is mediated by steroid hormones, BMP/Smad, and the MAPK-Ras pathways [107,108].
Communication between oocytes and granulosa cells is the cause of establishment of the microenvironment for oocytes. By command of an oocyte-secreted morphogenic gradient, the tertiary follicle's granulosa cells begin to differentiate themselves into four distinct subtypes: the corona radiata that surround the zona pellucida, the membrana that is interior to the basal lamina, the periantral that is adjacent to the antrum, and the cumulus oophorus that connects the membrana and corona radiata granulosa cells together. Grondahl et al. [109] identified a total of 1,562 genes that are differentially expressed by >2-fold in the cumulus cells and mural granulosa cells, and demonstrated the specialized function of these cells.
In vitro tertiary follicle culture to obtain competent oocytes
In many fundamental studies the regulation mechanisms of tertiary follicle growth have been explored, but many issues, so far, remain unclear. One of the reasons is the complexity of in vivo regulation mechanisms and and their system. Dilution of the effect after treatment is the first error during in vivo study. From the study of Eppig and Schroeder [110], in vitro culture systems of follicles have been designed and examined. Eppig and Schroeder [110] were able to obtain living offspring from in vitro matured oocytes that were derived from preantral follicles. In 1996, Eppig and O'Brien [111] obtained better frequency of mature oocytes and mouse offspring using a two-step strategy. A few studies have shown successful folliculogenesis after growth on a biomatrix [112-114] and various culture devices [115,116]. Kreeger et al. [117] used an alginate-extracellular matrix gel scaffold containing defined extracellular matrix (ECM) compounds for the culture of follicles and then produced a steroid synthetic follicle culture system. However, they need a large number of pre-culture procedures and their production and testing capacity was too low. However, from their results it is suggested that the effects of ECM components in the scaffold depend upon both the components of the ECM and the initial culture stage of the follicles. The recently tested hyaluronan-based hydrogel scaffolding of follicles has limitations. The matrix mixed hydrogel scaffold also has limitations. Although the architecture of the follicles was maintained by hydrogel embedding, this did not increase the proportion of competent oocyte production [114].
On the other hand, tertiary stage folliculogenesis is critical in the maturity and competence of ovulated oocytes. Therefore, it is critical to understand the growth regulation and growth mechanisms of tertiary follicles. In addition it is critical to evaluate the relationship among gonadotropin, follicle cells, and grown oocytes. Progress toward developing a culture system that allows the complete growth of early tertiary follicles to rupture and oocyte maturation remains a major challenge. In one of our previous studies, we used the modified hanging-drop method with simple media, no antibiotics or scaffold, and gonadotropin-induced follicles. This method is simple compared to the methods using culture devices or scaffolds. Early tertiary follicles were isolated from the ovary with 27-G needles. Follicles were individually cultured for 39 hours at one follicle per well of a 96-well plate. The plates were inverted prior to incubation (Figure 2). The modified hanging-drop culture is as effective as the inverted culture of Nation and Selwood [115] for obtaining ovulated oocytes [118]. The growth of early tertiary follicles is improved by this method and the number of ovulated oocytes is also higher [118] (Figure 2). Based on this system, RNAi or other functional modulators were successfully and easily applied at cell or tissue levels [118]. Based on these studies, it is suggested that this modified hanging-drop culture method will be useful to study folliculogenesis in vitro and may be applied to assisted reproductive technology (ART). Although further optimization of the culture and scaffolding environment is necessary to obtain competent oocytes, in vitro culture technologies are opening new avenues toward assisted reproduction.
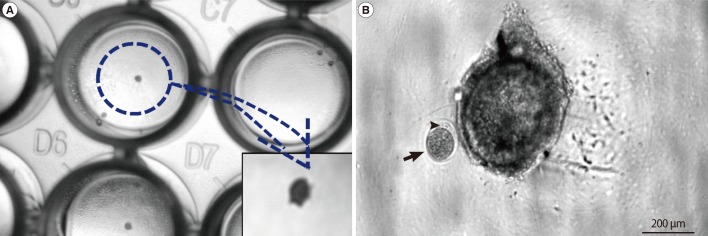
Modified hanging-drop culture of early tertiary follicles. (A) Light micrograph of tertiary follicle culture. An early tertiary follicle was dropped in one well of a 96-well tissue culture plate, and the plate was reversed and cultured. (B) Light micrograph of ovulated mature oocyte. After 40 hours culture, the oocytes were ovulated. The arrow indicates the ovulated oocyte and arrow head points to the first polar body.
Conclusion
Although the development of tertiary follicles is necessary, it is not sufficient for fertilization. In the end, the grown follicles have to be selected to attend ovulation. It has been suggested that changes in intrafollicular activins, GDF-9, AMH, and several BMPs contribute to this selection process by modulating both FSH- and IGF-dependent signaling pathways in granulosa cells [119]. It is known that almost 90% of the initial pool is depleted by puberty in humans, and beyond age 37, the number of follicles approaches approximately 25,000 follicles and the rate of follicle loss increases so that by age 50, approximately 1,000 follicles remain [120,121]. Over the past two decades, numerous mouse models have been generated that demonstrate the importance of multiple signaling systems in follicle growth. Studies using mice provide a basis for understanding human infertility and disease as well as revealing new candidate genes for mutational analysis. However, it is not enough to evaluate the mechanisms of folliculogenesis. As a new approach, the in vitro follicle culture model is useful for exploring the phenomena in the growth and differentiation of follicles and preparation for the ovulation of oocytes and fertilization. Such approaches will accelerate resolution of the questions involved in folliculogenesis and in obtaining competent oocytes. By refining these skills, ART can be assisted to govern the regulators in insuring the maintenance of reproductive ability.
Notes
This work was supported by Sungshin Women's University.
No potential conflict of interest relevant to this article was reported.