Preliminary clinical outcome of novel strategy for the maximization of cumulative pregnancy rates per retrieval in normal responders
Article information
Abstract
Objective
We devised a novel strategy, a GnRH antagonist protocol with a GnRH agonist trigger followed by frozen-thawed blastocyst transfers with long zona dissection (LZD). The purpose of this study was to investigate the clinical outcomes of this new strategy according to age.
Methods
Ninety women aged less than 35 (group A) and 32 women aged 35 to 39 (group B) underwent the GnRH antagonist protocol with a GnRH agonist trigger in order to obtain many oocytes and prevent early-onset ovarian hyperstimulation syndrome (OHSS). All oocytes were cultured to the blastocyst stage and all blastocysts grade 3BB or better were cryopreserved. Embryo transfers were only performed in freeze-thaw cycles to prevent late-onset OHSS and to overcome embryo-endometrium dyssynchrony. LZD was performed just after thawing to improve hatching and implantation rates.
Results
The average numbers of retrieved oocytes and blastocysts grade 3BB or better were 12.8±5.5 and 4.4±2.6 in group A and 10.9±7.4 and 2.5±2.2 in group B, respectively, and OHSS did not occur in any of the women. Implantation rates were 46.7% in group A and 39.3% in group B. Cumulative clinical pregnancy rates per retrieval were 77.8% in group A and 62.5% in group B. Cumulative ongoing pregnancy rates per retrieval were 71.1% in group A and 53.1% in group B.
Conclusion
GnRH antagonist protocol with GnRH agonist trigger followed by frozen-thawed blastocyst transfers with LZD can generate many blastocysts without OHSS and maximize cumulative pregnancy rates per retrieval. This strategy is more effective in young women aged less than 35 than in women aged 35 to 39.
Introduction
Theoretically, if any IVF strategy simultaneously satisfies the maximization of the cumulative pregnancy rate per retrieval, the prevention of ovarian hyperstimulation syndrome (OHSS), the prevention of multiple pregnancy, and the minimization of inconvenience and cost, it may be one of the most ideal methods. The controlled ovarian stimulation (COS) method, which can produce many oocytes without OHSS, and the freezing-thawing method, which can assure high survival, hatching, and implantation rates, will be needed in order to accomplish these aims.
In the last decade, the clinical outcomes of frozen-thawed embryo transfers were inferior to fresh embryo transfers. However, recently, with the developments of vitrification and assisted hatching (AH), frozen-thawed embryo transfers showed prominently improved results [1]. In fact, it has even been reported that the clinical outcomes of frozen-thawed embryo transfers are superior to fresh embryo transfers owing to better embryo-endometrium synchrony [2,3]. It has been well known that premature progesterone elevation and embryo-endometrium dyssynchrony in COS cycles impair implantation and no pregnancies are achieved when the endometrium is advanced for more than 3 days [4,5]. If any freezing-thawing method assures high survival, hatching, and implantation rates for embryos, frozen-thawed embryo transfers can routinely substitute for fresh embryo transfers. GnRH antagonist protocol with GnRH agonist trigger is not commonly used because of severe luteal phase defects and a low pregnancy rate, except in patients with high risk of OHSS [6]. Nevertheless, this protocol could be very effective in patients planning for frozen-thawed blastocyst transfers, because it can simultaneously accomplish the collection of many oocytes and the prevention of early-onset OHSS.
Therefore, we devised a novel strategy that combined several methods as follows: 1) GnRH antagonist protocol with GnRH agonist trigger to obtain many oocytes and to prevent early-onset OHSS, 2) blastocyst culture to screen arrested embryos or low-quality blastocysts out, 3) vitrification with artificial shrinkage to improve the survival rate of embryos, 4) frozen-thawed embryo transfers to prevent late-onset OHSS and to synchronize between embryos and the endometrium, and 5) assisted hatching (AH) to overcome zona hardening. We investigated the clinical outcomes of this new strategy according to age in normal responders.
Methods
1. Patients
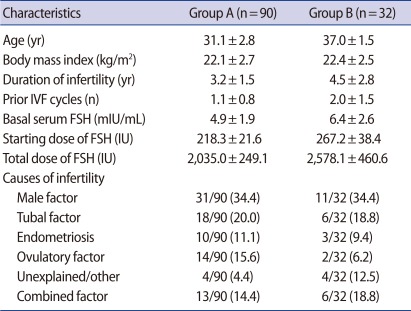
A total of 90 women aged less than 35 (group A) and a total of 32 women aged 35to 39 (group B) were included in this study from July 2009 to December 2010. The inclusion criteria of patients were defined as follows: 1) basal follicle-stimulating hormone (FSH) level less than 10 mIU/mL, 2) normal uterine cavity, and 3) adequate sperm for IVF or ICSI. Before controlled ovarian stimulation, all women had their endometrial and tubal pathology evaluated by hysteroscopy or hysterosalpingography, and if necessary, endometrial or tubal surgery were performed.
2. Controlled ovarian stimulation
GnRH antagonist protocol with GnRH agonist trigger was used preceded by an oral contraceptive preparation for 2 to 3 weeks. 150-450 IU/day of recombinant FSH (Gonal-F, Merck Serono, Darmstadt, Germany) was administrated from cycle day 2 or 3 and individualized according to the antral follicle count, basal FSH, age, and body weight. When leading follicles reached the mean diameter of 13 to 14 mm, the GnRH antagonist of cetrorelix 0.25 mg/day (Cetrotide, Merck Serono) was administrated. In case folliclular development had been slow or insufficient, 75 to 150 IU/day of recombinant LH (Luveris, Merck Serono) or hMG (Menopur, Ferring, Lausane, Switzerland) were added. When the leading follicles reached the mean diameter of 17 to18 mm, GnRH agonist triptoreline 0.2 mg (Decapeptyl, Ferring) was administrated for trigger of ovulation. Oocyte retrieval was performed transvaginally under ultrasound guidance 35 to 36 hours after GnRH agonist trigger.
3. Blastocyst culture and selection
After standard IVF or ICSI, fertilization was assessed 15-18 hours after insemination by the presence of two pronuclei. All embryos were cultured in sequential media G1/G2 in a 6% CO2, 5% O2, and 89% N2 environment [7].
All blastocysts were evaluated using Gardner and Schoolcraft's scoring system [8]. In this study, blastocysts more than grade 3BB were selected for cryopreservation and embryo transfers.
4. Vitrification and thawing
The vitrification solution (G25E25) was composed of 25% glycerol (Sigma) + 25% ethylene glycol (Sigma) + 20% synthetic serum substitute (SSS) in Dulbecco's phosphate buffered saline (DPBS) (Gibco, Carlsbad, CA, USA). The equilibration solution (G10 and G10E20) was composed of 10% glycerol and 10% glycerol + 20% ethylene glycol in DPBS with 20% SSS. Artificial shrinkages of all blastocysts grade 3BB or better were performed with ICSI pipettes, as we described previously (Figure 1) [9]. After artificial shrinkage, the blastocysts were equilibrated in G10 and G10E20 at room temperature for 3 minutes, respectively, and exposed in G25E25 for a maximum of 30 seconds. An approximate volume of 0.3 µL of vitrification solution containing blastocysts (maximum 2) was loaded on the self-manufactured "capped-pulled straw", as previously described [9]. After holding on liquid nitrogen (LN2) vapor for 10 seconds, the capped-pulled straw was plunged into LN2.

Artificial shrinkage using ICSI pipettes. (A) Locate inner cell mass in the 6 or 12 o'clock direction and hold a blastocyst. (B) Insert injection pipette from the 3 o'clock direction. (C) Aspirate blastocoel fluid. (D) Blastocyst after artificial shrinkage.
Thawing was performed in 1 mL of 0.5, 0.25 mol/L sucrose and DPBS with 10% SSS. The tip of the pulled straw was immersed into 1 mL of 0.5 mol/L sucrose at room temperature. After 3 minutes, the blastocysts were transferred into 0.25 mol/L sucrose and DPBS with 10% SSS at room temperature for 3 minutes each. After washing three times in 37℃ DPBS with 10% SSS, AH was performed.
5. Long zona dissection as AH
AH was performed by long zona dissection (LZD) using ICSI pipettes in the process of thawing (Figure 2). Briefly, a frozen-thawed blastocyst was held by holding a pipette in the 9 o'clock direction and then the zona pellucida (ZP) was completely penetrated by injection pipette from the 3 o'clock direction to the 9 o'clock direction. Thereafter, a large split was created by LZD using holding and injection pipettes.
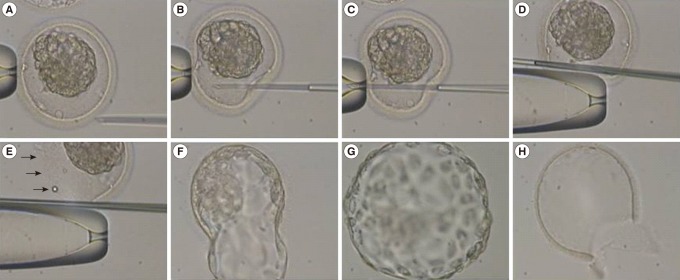
Long zona dissection using ICSI pipettes just after thawing. (A) Hold a blastocyst in the 9 o'clock direction. (B) Insert the injection pipette from the 3 o'clock direction. (C) Penetrate through the perivitelline space to the 9 o'clock direction. (D) Rub and flick the zona pellucida (ZP) using holding and injection pipettes. (E) Dissected ZP (arrows). (F) Hatching blastocyst. (G) Hatched blastocyst. (D) Remnant ZP after hatching.
6. Endometrial preparation and blastocyst transfer
Oral contraceptives was administrated from the preceding menstrual cycle day 2 to 4 and leuprolide acetate 0.5 mg/day (Lucrin, Abbott, IL, USA) was administrated from the preceding menstrual cycle day 21 with a 5-day overlap of the GnRH agonist and oral contraceptives. The suppressed serum progesterone and the early proliferative endometrial pattern on transvaginal ultrasound were confirmed before sequentially increasing the doses of oral estradiol valerate (Progynova, Bayer Schering, Leverkusen, Germany). In general, oral estradiol valerate (2 mg) was administrated once a day on cycle days 2 to 5, twice a day on cycle days 6 to 9, and three times a day on cycle days 10 to14. In case endometrial thickness on transvaginal ultrasound was less than 7 mm by cycle day 14, oral estradiol valerate (2 mg) four times a day was administered. For luteal support, oral estradiol valerate (2 mg) twice a day and vaginal progesterone (Crinone gel 8%, Merck Serono) once a day were administrated from cycle day 15. Frozen-thawed blastocysts were transferred on cycle day 20 or 5 days after the start of luteal support. The number of transferred blastocysts, ranging from one to three, was decided by the patient.
7. Outcome measures and statistical analyses
Implantation and clinical pregnancy were defined as the presence of a gestational sac with cardiac activity at 6 weeks of pregnancy. Ongoing pregnancy was defined as the presence of a gestational sac with cardiac activity at 12 weeks of pregnancy. OHSS was identified by the presence of ascites on day 3 after oocyte retrieval. The clinical outcomes of frozen-thawed blastocyst transfers were analyzed by the Student's t test, chi-squared test, or Fisher's exact test. A value of p less than 0.05 was considered to be statistically significant. Data are expressed as mean±SD unless otherwise specified.
Results
Table 1 shows the patient characteristics of group A (women aged less than 35) and group B (women aged 35-39).
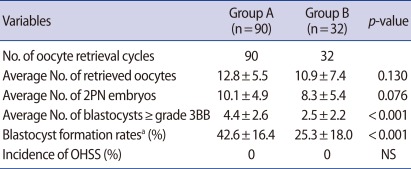
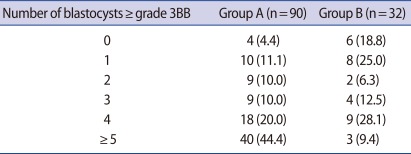
Table 2 shows the clinical outcomes for the GnRH antagonist protocol with a GnRH agonist trigger. The average numbers of retrieved oocytes and 2 PN embryos were 12.8±5.5 and 10.1±4.9 in group A and 10.9±7.4 and 8.3±5.4 in group B, respectively. The average numbers and average formation rates of blastocysts grade 3BB or better were 4.4±2.6 and 42.6±16.4% in group A and 2.5±2.2 and 25.3±18.0% in group B, respectively. OHSS did not exist in any of the patients on day 3 after oocyte retrieval. Group A showed higher trends than group B in terms of all variables, except incidence of OHSS. In particular, the average numbers and average formation rates of blastocysts grade 3BB or better were significantly higher in group A than group B (p less than 0.05). Table 3 shows the distribution of patients according to the number of blastocysts grade 3BB or better. In group A, 4.4% (4/90) of the patients had no blastocysts as did 18.8% (6/32) in group B. In group A, 11.1% (10/90) of the patients had 1 blastocyst and in group B 25.0% (8/32) did. In group A, 64.4% (58/90) of the patients had more than 4 blastocysts as did 37.5% (12/32) in group B.
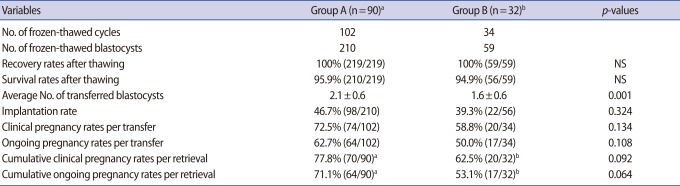
Table 4 shows the clinical outcomes for frozen-thawed blastocyst transfers. The average recovery and survival rates of frozen-thawed blastocysts were 100% and 95.9% in group A and 100% and 94.9% in group B, respectively. The average numbers of transferred blastocysts of grade 3BB or better were 2.1±0.6 in group A and 1.6±0.6 in group B. Implantation rates were 46.7% in group A and 39.3% in group B. Clinical pregnancy and ongoing pregnancy rates per transfer were 72.5% and 62.7% in group A and 58.8% and 50.0% in group B, respectively. Cumulative clinical pregnancy and cumulative ongoing pregnancy rates per retrieval were 77.8% and 71.1% in group A and 62.5% and 53.1% in group B, respectively. Group A showed the higher rates than group B for all variables, except recovery and survival rates, as well as average numbers of transferred blastocysts of grade 3BB or better.
Discussion
Our strategy combined several methods as follows: 1) A GnRH antagonist protocol with a GnRH agonist trigger, 2) blastocyst culture and selection, 3) frozen-thawed blastocyst transfers, 4) vitrification with artificial shrinkage, and 5) LZD as AH.
Firstly, the GnRH antagonist protocol with GnRH agonist trigger was used for the prevention of early-onset OHSS and the acquisition of many oocytes. A GnRH agonist trigger is not generally used because of severe luteal phase defects and low pregnancy rates, but this disadvantage can be transformed into an advantage in patients planning for frozen-thawed blastocyst transfers [6]. This protocol is very useful in high responders, especially in patients with polycystic ovary syndrome. Even though early-onset OHSS occurs temporarily in patients with severe polycystic ovary syndrome, it disappears rapidly after oocyte retrieval owing to the short effect of GnRH agonist. In addition, this protocol is superior to IVM with regard to the numbers of mature oocytes and blastocysts. In this study, we use GnRH agonist for ovulation triggering, but in other cases like low responding or old age groups, we recommend hCG for ovulation triggering.
These advantages confirmed in the results of our study that the average numbers of retrieved oocytes and blastocysts that were grade 3BB or better were 12.8±5.5 and 4.4±2.6 in women aged less than 35 (group A) and 10.9±7.4 and 2.5±2.2 in women aged 35-39 (group B), respectively, and that OHSS did not exist in any of the patients on day 3 after oocyte retrieval. Theoretically, when the implantation rate is 46.7%, the patients with more than four blastocysts can achieve a cumulative clinical pregnancy rate per retrieval of approximately 92%. In reality, 64.4% (58/90) of the patients of group A showed cumulative clinical pregnancy rate of approximately 96% per retrieval. Therefore, the COS method, which can procure many oocytes without OHSS, is very important in order to maximize the cumulative clinical pregnancy rate per retrieval.
The potential advantages of blastocyst culture are to screen aneuploidic or arrested embryos out, to synchronize the embryonic stage with the uterine environment, and to reduce the chance of embryonic expulsion from the uterus [7,10,11]. Also, cryopreservation is more successful at the blastocyst stage than earlier stages [12]. Above all, in our experience, good-quality embryos in the cleavage stage do not grow into blastocysts of grade 3BB or better and are frequently arrested in the cleavage and morula stage.
Gardner et al. [13,14] have reported the clinical outcomes of fresh blastocyst transfer cycles according to blastocyst score. Implantation and clinical pregnancy rates were 69.9% and 86.8% in the group of 2 blastocysts grade 3AA or better transferred and were 56.0% and 76.0% in the group of two blastocysts grade 3BB or better transferred, respectively. Recently, Goto et al. [1] have reported the clinical outcomes of 1,488 single frozen-thawed blastocyst transfers according to blastocyst score and age. The implantation rates were 50.5% to 70.1% in the groups of one blastocyst grade 4BB or better transferred and were 45.6% to 51.7% in the groups of one blastocyst of grade 3AA, 3AB, or 3BA transferred, respectively. These reports showed that implantation rates differ according to the blastocyst score and age. In our study, the selected blastocysts were at least grade 3BB and implantation rates were 46.7% in women aged less than 35% and 39.3% in women aged 35 to 39. Goto et al. [1] and our studies showed that frozen-thawed blastocysts can provide high enough implantation rates.
Frozen-thawed blastocyst transfers were used for the prevention of delayed-onset OHSS and better embryo-endometrium synchrony. It has been well known that no pregnancies are achieved in cases of endometrial advancement exceeding 3 days in COS cycles [5]. The cryopreservation of all blastocysts has several advantages owing to a lack of demand for embryo-endometrium synchrony in the COS cycle. All of the good-quality blastocysts that develop on day 5 or day 6 can be always transferred under complete embryo-endometrium synchrony. In COS cycles, the implantation rate for day 6 blastocysts is lower than that of day 5 blastocysts; however, in frozen-thawed cycles, the clinical outcomes of day 6 blastocysts are similar to day 5 blastocysts [15]. Also, in donor-recipient cycles, the complex processes for embryo-endometrium synchrony are not required. With the complete separation between oocyte retrieval and embryo transfer, COS and monitoring are very simple and free from the control of the gonadotropin dose as well as the timing of GnRH antagonist and ovulation trigger. Transvaginal ultrasound alone is enough to monitor COS cycles.
Earlier, we reported vitrification with artificial shrinkage using ICSI pipettes, which showed a high survival rate of more than 90% in mouse expanding blastocysts [9]. Our study showed that the recovery rate was 100% and survival rate was approximately 95%. The recovery and survival rates prominently affect the cumulative pregnancy rates per retrieval, especially in patients with small numbers of blastocysts; therefore, the high recovery and survival rates are required for the strategy of frozen-thawed blastocyst transfers in all patients.
LZD using an ICSI pipette as mechanical AH was used to overcome zona hardening. LZD is very important for achieving a high implantation of blastocysts. LZD or controlled zona dissection was first reported in mouse and human fresh embryos by Lyu et al. [16]. In 8-cell mouse embyos, LZD with a large slit (beyond two-thirds of the zona diameter) significantly enhanced complete hatching rates compared to three-dimensional partial zona dissection and no AH: 93.9% vs. 62.0% vs. 38.8%, respectively. In human blastocysts, LZD with a large slit significantly enhanced complete hatching rates compared to a moderate slit (two-fifths of ZP diameter): 100% vs. 71.8%, respectively. They demonstrated that the complete hatching rate of the AH group is higher than the no AH group and a hole size created on ZP is important for complete hatching because the zona opening of small or moderate size can often trap the ICM. Also, we havepreviously reported the effects of LZD using ICSI pipettes in frozen-thawed mouse blastocysts (Jo et al. [9]). Complete hatching rates were significantly higher in the AH group than the no AH group: 100% vs. 50%, respectively. These reports showed that the complete hatching rates of the no AH group is very low in frozen-thawed cycles as well as fresh cycles and LZD can completely overcome zona hardening.
In our study, LZD were performed just after thawing in order to prevent potential contamination or infection during in vitro culture and cryopreservation. Compared with other methods, this method has a small variation regarding clinical outcomes and a large slit could be easily created by an embryologist handling a micromanipulator owing to the wide perivitelline space around the shrunken blastocyst.
This novel strategy can resolve many problems that are currently faced in IVF programs, and can simplify the complex processes related to COS and monitoring. In addition, this strategy can simultaneously satisfy the ideal aims of IVF program such as the maximization of the cumulative pregnancy rate per retrieval, the prevention of OHSS in high responders such as patients who had polycystic ovarian syndrome and multiple pregnancy, and the minimization of patients' inconvenience and cost. In particular, this strategy is more useful in young women aged less than 35 than it is in older women aged 35 to 39.
This study was limited by a small sample size and retrospective design. However, it shows the need for further case-controlled prospective studies.
Notes
This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A090602).
No potential conflict of interest relevant to this article was reported.