Effect of artificial shrinkage on clinical outcome in fresh blastocyst transfer cycles
Article information
Abstract
Objective
This study aimed to determine the safety and clinical effect of artificial shrinkage (AS) in terms of assisted hatching of fresh blastocysts. Also, we evaluated the correlation between patient age and the effect of AS on clinical outcome.
Methods
Two AS methods, using a 29-gauge needle and laser pulse, were compared. Seventy-three blastocysts were shrunk using a 29-gauge needle and the same number of other blastocysts were shrunk by a laser pulse. We evaluated the shrunken blastocysts hourly and considered them viable if they re-expanded >70%. Blastocyst transfer cycles (n=134) were divided into two groups: a control group consisted of the cycles whose intact embryos were transferred (n=100), while the AS group consisted of the cycles whose embryos were replaced following AS (n=34). The implantation and pregnancy rates of the control group and AS group were compared (p<0.05).
Results
The re-expansion rates of the 29-gauge needle and laser pulse AS groups were similar (56 [76.7%] vs. 62 [84.9%], respectively). All of the remaining shrunken blastocysts were re-expanded within 2 hours. There was no degeneration of shrunken blastocysts. The total and clinical pregnancy rate of the AS group (23 [67.6%]; 20 [58.8%], respectively) was significantly higher than that of the control group (47 [47.0%]; 39 [39.0%], respectively). In the older patient group, there was no difference in the clinical outcomes between the AS and control groups.
Conclusion
These results suggest that AS of blastocoele cavity, followed by the transfer, would be a useful approach to improve the clinical outcome in cycles in which fresh blastocyst stage embryos are transferred.
Introduction
In developing from the morula to the blastocyst, embryos undergo dramatic morphologic changes. When blastocysts expand, fluid gradually accumulates in the blastocoel-mediated sodium pump (Na+, K+, ATPase) [1], resulting in increased pressure on both the trophectoderm and zona pellucida (ZP). At the same time, trophectoderm cells secrete lysins that are involved in ZP thinning and hatching. Expansion and ZP thinning occur in mammalian blastocysts prior to hatching [2-4]. Contraction-expansion cycles, as well as expansion and ZP thinning of blastocysts, have been documented by time-lapse video recording [5,6].
The rationale of artificial shrinkage (AS) is based on contraction-expansion cycles and cytoplasmic extension of the trophectoderm of the blastocyst. The mechanism of blastocoel collapse and recovery of trophectoderm rupture is unclear. Whatever causes a collapse of the blastocyst, shrunken blastocysts have the potential to gradually recover their spheroidal shape. The AS technique is widely used and considered an essential step to improve the efficiency of vitrification. Researchers using AS before vitrification of blastocysts have reported higher implantation and clinical pregnancy rates [7-12].
The advantage of AS is that ice crystal formation can be avoided by reducing the fluid content of the blastocoel [7]. Some researchers assume that creating a large hole in the ZP with any of various AS tools (i.e., glass micro-needle, injection needle, micropipetting with a hand-drawn Pasteur pipette, 29-gauge needle, or laser pulse) has some kind of effect on assisted hatching (AH) [13,14]. In the process of AS, various AS tools create physical damage to the ZP as well as to the trophectoderm. It is assumed that re-expanding the blastocyst during warming eases hatching through the ZP-damaged areas. In fact, most thawed blastocysts with AS have a higher hatching rate, whether or not they hatch through the ZP-damaged areas.
Although blastocyst transfer has provided good clinical results, researchers have become increasingly aware that it is more difficult to obtain a certain pregnancy rate [15,16]. Thus, some researchers have attempted to include an additional process before blastocyst transfer to achieve a higher pregnancy rate. Fong et al. [17] attempted to remove the total ZP with pronase before blastocyst transfer, Turker [18] attempted to make a large hole in the ZP with acid Tyrode's solution, and Goto et al. [19] attempted to stimulate the endometrium by injection of embryo culture supernatant into the uterus. We attempted to use the AS technique on fresh blastocysts before transfer.
The objective of this study was to determine the impact of AS on fresh blastocysts, and to determine whether or not AS had an effect, such as assisted hatching, by comparing clinical outcomes. We evaluated the correlation between patient age and the effect of AS on clinical outcome.
Methods
1. Patients and IVF
Patients who entered our blastocyst transfer program and agreed to perform assisted hatching were≥36 years of age or had previous repeat failures of cleavage-stage embryo transfers. Between July and December 2008, 100 cycles were randomly selected as a control group. We applied the 29-gauge needle AS technique to 34 cycles. In each group (control and AS groups), we evaluated the effect of the AS technique on patients ≥36 years of age. Patients were treated with GnRH agonist and hMG in according to a long- or a short-treatment protocol. When two and more follicles reached 18 mm in diameter, 5,000 IU of hCG (Ovidrel; Merk-Serono, Bari, Italy) was administered. Oocytes were retrieved transvaginally 36-38 hours after hCG injection and the oocytes were inseminated by conventional IVF or ICSI.
2. Embryo culture
Fertilization was assessed 15-18 hours after insemination by the presence of two pronuclei. Zygotes were washed and cultured in groups <5 in Sydney IVF Cleavage Medium (CM; COOK, Brisbane, Australia) for 48 hours, then in Sydney IVF Blastocyst Medium (BM; COOK) for another 48-71 hours. All culturing of embryos was performed in a CO2:O2:N2 (6%:5%:89%) environment. On day 5, blastocysts were scored depending on the developmental stage and graded according to quality criteria [20].
3. Artificial shrinkage of expanded blastocysts
Son et al. [8] previously described the mechanical technique of shrinkage using two 29-gauge needles. Briefly, after holding the expanded blastocyst with the flat side of a needle and placing the inner cell mass (ICM) at the 12 or 6 o'clock position, a needle was pushed through the trophectoderm cell in the blastocoel cavity until the cavity shrank. Contraction of the blastocysts was observed after 30 seconds to 1 minute. After complete shrinkage of the blastocoel, the blastocysts were cultured in BM before embryo transfer.
A laser system (ZILOS-tk Laser Zona Drill System; Hamilton Thorn Bioscience, Inc., Beverly, MA, USA) was introduced to compare with the 29-gauge needle AS. The process of the AS was followed by Mukaida's method [14]. The ICM should be placed away from the targeted point of the laser pulse. A single laser pulse (300 µs) was delivered to the outer periphery of the trophectoderm at the junction between the cells. It was sufficient to induce complete shrinkage within 1-2 minutes. After complete shrinkage of the blastocoel, the blastocysts were cultured in the same media.
4. Evaluation of impact of AS on fresh blastocysts
Several previous studies have concluded that AS before vitrification is a useful approach for improving clinical outcomes and guaranteeing the safety of the AS technique for human blastocysts. With a slightly different perspective, we attempted to determine the impact of AS on fresh blastocysts that were surplus embryos for cryopreservation. A 29-gauge needle and laser pulse AS were compared. Seventy-three blastocysts were shrunk by using a 29-gauge needle and the same number of other blastocysts were shrunk using a laser pulse. After AS was performed, every blastocyst was evaluated hourly until the re-expansion rate was up to 70%. We confirmed that 70% of the re-expanded blastocysts were viable. After observing for 2 hours, the highest quality blastocysts were cryopreserved and the others were discarded.
Assisted hatching of blastocysts and AS before vitrification of every blastocyst has already been permitted by the policy of the Maria Fertility Hospital Institutional Review Board. The evaluation of re-expanded blastocysts was treated as a part of the whole freezing process.
5. Blastocyst transfer and assessment of pregnancy
On the morning of embryo transfer (ET), we selected 2 or 3 good quality blastocysts and performed AS before transfer. After 2 or 2.5 hours, we selected the best quality re-expanded blastocysts and transferred them. We were limited to transfering two blastocysts. Only a few patients received three blastocysts, based on their clinical history (i.e., repeat failures or age ≥36 years). Pregnancy was assessed by serum hCG 14 days after administration of progesterone, then implantation was confirmed by the presence of a gestational sac. Clinical pregnancy was confirmed by the presence of fetal heart activity.
6. Statistics
The data were examined by χ2 analysis to determine whether differences in implantation and pregnancy rates were significant between the control and AS groups.
Results
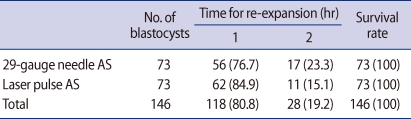
We evaluated the impact of AS on fresh blastocysts by observing the rate of re-expansion to 70% of the original volume. The 29-gauge needle and laser pulse AS were compared (Table 1). Seventy-three blastocysts were shrunk by using a 29-gauge needle; 56 blastocysts (76.7%) were re-expanded up to at least 70% within 1 hour (Figure 1). The remaining blastocysts (17 [23.3%]) were re-expanded within 2 hours.
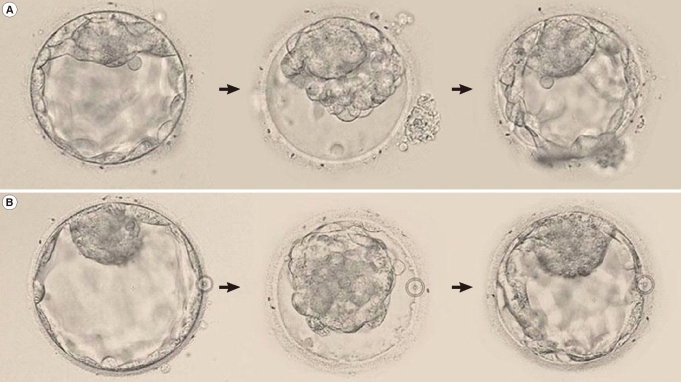
Blastocyst shrinkage and re-expansion (≥70%) using a 29-gauge needle (A) and laser pulse (B) (magnification×200).
The same number of other blastocysts were shrunk using a laser pulse; 62 blastocysts (84.9%) were re-expanded up to at least 70% within 1 hour. The remaining blastocysts (11 [15.1%]) were re-expanded within 2 hours. All blastocysts were re-expanded up to 70% within 2 hours. There was no degeneration of the shrunken blastocysts.
The re-expansion rates using the 29-gauge needle and laser pulse AS were similar. Nearly 80% of the shrunken blastocysts were re-expanded within 1 hour. All of the remaining shrunken blastocysts were re-expanded within 2 hours.
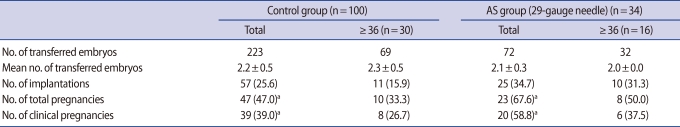
Based on previous results, we applied the AS technique clinically. Table 2 summarizes the clinical outcome comparing the control and AS groups. Laser pulse AS had not been applied by that time. The mean number of blastocyst transfers was quite similar between the control and AS groups. The implantation rate of the 29-gauge needle AS group was higher than the control group, but the difference was not statistically significant. The total and clinical pregnancy rates of the AS group were significantly higher than those of the control group. In patients with an age of ≥36 years, the clinical outcome of the AS group was higher than that of the control group, but the difference was not statistically significant.
Discussion
This study demonstrated that AS of fresh blastocysts before transfer had very encouraging clinical results. We confirmed the safety of laser pulse AS as well as 29-gauge needle AS on fresh blastocysts. Our laboratory has previously reported a vitrification method for human blastocysts on electron microscopy grids combined with AS using a 29-gauge needle [8], but we have also published serial studies which led to the previous study [21-24]. Thus, we have considerable experience with AS using a fine needle. Due to our accumulated experience, we reasoned that physical damage would be minimized.
As mentioned earlier, AS before vitrification of blastocysts and AH of blastocysts has already been permitted by the policy of the Maria Fertility Hospital Institutional Review Board. The evaluation of re-expanded blastocysts was treated as a part of the whole freezing process. Most of the blastocysts designated for vitrification were re-shrunk in the media for cryopreservation. Some blastocysts that did not shrink in the freezing media were cryopreserved without going through AS. We had experienced that sometimes a blastocyst was not shrink completely. We could not find the reason why some blastocysts shrink incompletely.
During embryo biopsy for pre-implantation genetic diagnosis (PGD), a large hole is created in the ZP of the blastocyst. However, the safety of biopsy for PGD is still a matter of debate [25-27]. It appears that the effect of AS is less extensive than PGD by comparing several clinical outcomes. We recommend that additional study is needed at the DNA/cellular level to determine the effects of AS on fresh blastocysts.
The advantage of AS is improving blastocyst viability after vitrification, which prevents ice crystal damage by reducing fluid within the blastocoel cavity [7]. Other researchers have estimated that there may be an effect of assisted hatching caused by the formation of a large hole in the ZP, especially with 29-gauge needle AS [8]. We have confidence that there is a helpful effect of AS in terms of AH. Most previous reports on the effect of AH have performed AH on the cleavage stage using acid Tyrode's solution, a mechanical method, or a laser [28,29]. Enzymatic treatment of the ZP to completely remove the ZP before blastocyst transfer has resulted in a high implantation rate [30]. AH at the blastocyst stage needs a more careful approach. Some researchers claim that there is no need to do AH on blastocysts because they have an intrinsically higher viability than cleavage-stage embryos [31]. Our study showed that the AS technique is not harmful to the survival rate of blastocysts, and is worthwhile as a new AH technique to perform on blastocysts before transfer.
Single embryo transfer (SET) is the effective method for reducing the IVF multiple pregnancy rate [32]. For SET to be successful, it is crucial to have confidence in embryo viability [33]. Blastocyst transfer is a better method for diminishing the controversy over embryo viability. Combining a single blastocyst and the AS technique before transfer would be a better approach to SET.
In this study, the tendency for AS to improve the rate of blasctocysts having successful implantation and pregnancy in patients aged ≥36 years was insignificant. Further study is needed to determine whether or not AS is helpful in older patients.
In conclusion, this study showed that AS of human blastocysts before transfer is a clinically useful method.
Notes
Presented at 20th World Congress of Fertility and Sterility, Munich, Germany.
No potential conflict of interest relevant to this article was reported.