Functions of PIWI proteins in spermatogenesis
Article information
Abstract
Recently, a significant understanding of the molecular mechanisms regulating spermatogenesis has been achieved utilizing small RNA molecules (small RNAs), including small interfering RNAs (siRNAs), microRNAs (miRNAs), and Piwi-interacting RNAs (piRNAs) which emerged as important regulators of gene expression at the post-transcriptional or translation level. piRNAs are only present in pachytene spermatocytes and round spermatids, whereas miRNAs are expressed abundantly in male germ cells throughout spermatogenesis. This review is aimed at providing a glimpse of piRNAs and their interacting family proteins such as PIWIL1, PIWIL2, and PIWIL4 in spermatogenesis.
Spermatogenesis and piRNA regulation
Spermatogenesis is the process of the development of sperm from undifferentiated germ cells. It involves successive mitotic, meiotic, and post-meiotic phases. During this complex process, round undifferentiated spermatogonia become elongated, terminally differentiated spermatozoa, which are highly specialized cells responsible for delivering the paternal genome to the oocyte. Spermatogenesis requires a precise and well-coordinated system that regulates constantly changing patterns of gene and protein expression [1,2]. Small RNAs are important because of their diverse biological functions as potent regulators of transcription, RNA stability, and translation [3]. Classification of small RNAs is based upon their biogenesis, mechanisms of action, and functions [4], and there are three major small RNAs: small interfering RNAs (siRNAs), microRNAs (miRNAs), and Piwi-interacting RNAs (piRNAs). Recent studies have been successful in advancing current knowledge about small RNAs to the extent that they play major roles in the control of gene expression.
The genomic integrity of the germline is of fundamental importance for maintaining individuals and species, and a predominant biological factor that causes genome mutations is transposable elements [5]. Transposable elements and their fossil sequences occupy 46% and 39% of the genomes of humans and mice, respectively [6,7]. Line-1, the most abundant class of autonomous retrotransposons, has more than 500,000 copies in the haploid genome of mammals, and although most of them are truncated and nonfunctional, about 100 and 3,000 are active in humans and mice, respectively [8]. Line-1 insertions are estimated to account for about 0.1% of human mutations, and one in every 50 individuals has a new integration [8]. To control such mutagenic mobile elements, host genomes have evolved molecular defense systems. One key pathway, in mammals, is genome DNA methylation. Mutations in Dnmt1, a DNA methyl transferase, lead to retrotransposon deregulation and embryonic lethality [9,10], whereas Dnmt3L plays a specific role in suppressing retrotransposons in the germline [11]. Another critical and adaptive mechanism is RNA interference (RNAi) and its related systems, which utilize small noncoding RNAs that guide the effector complex, including argonaute proteins, to degrade and/or suppress target mRNAs. Germ cells are equipped with specific members of the argonaute subfamily, the PIWI proteins, which interact with piRNAs [12-15].
Discovery of PIWI proteins
The discovery of PIWI proteins and, in particular, piRNAs has revealed a new dimension of gene regulation. Several aspects of piRNA regulation change significantly during development. piRNAs, a class of small RNAs expressed in mammalian testis during spermatogenesis, interact with PIWI-family proteins such as MIWI, MIWI2, and MILI [12-14,16,17] (Figure 1). PIWI-family proteins refer to a group or family of proteins that have amino acid sequences, which are substantially identical to the native amino acid sequences in the PIWI family, and they include PIWI, HIWI, MIWI, MIWI2, MILI, PRG-1, and PRG-2 proteins. Both piRNAs and miRNAs are endogenous small RNAs, but piRNAs are distinct from miRNAs in their length and expression patterns [14]: they are 24-30 nt in length, and they are present in pachytene spermatocytes and spermatids during spermatogenesis, and required for germline development in both males and females [18]. It is estimated that the total number of piRNAs is around 2×105, suggesting that piRNAs may be essential for a broad range of biological processes [18,19]. It is intriguing that they are just a couple of nucleotides longer than the much better investigated and widely known miRNAs and siRNAs and also that their role appears to be silence retrotransposons, repetitive elements, and heterochromatic regions.
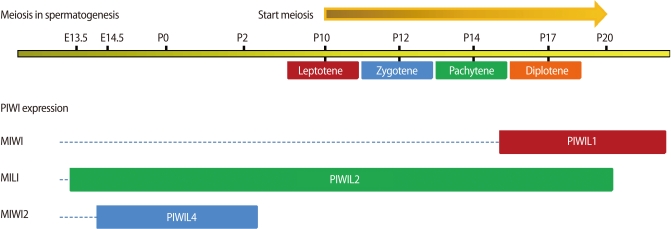
A diagram of expression of PIWI proteins and meiosis in spermatogenesis. PIWI expression is detectable at early embryonic day. PIWI proteins seem to take over the regulation of piRNA during meiosis in spermatogenesis. E, embryonic day; P, postnatal day (This figure is adapted from the reviews by Thomson and Lin [53], and Tushir et al. [54].).
Genetic studies in Drosophila have, initially, identified several genes that are involved in germ cell development and germline stem cell division. The former include the Oskar, Vasa, Nanos, Tudor, and Germ Cell-less genes [20], and the latter include the Piwi [21] and Yb [22] genes. However, the homologs of these gene products are not necessarily functional in the mammalian germ cell system. Gene targeting analyses of Mvh (mouse vasa homolog; Ddx4 - Mouse Genome Informatics, MGI), Mgcl-1 (mouse germ cell-less-1; Gcl - MGI) and Miwi (mouse piwi; Piwil1 - MGI) have shown that although these genes are dispensable for primordial germ cell (PGC) formation, they are essential for spermatogenesis [23-25]. In Drosophila, the loss of piwi function leads to the failure of germline stem cell self-renewal as well as downstream gametogenic functions such as germline cyst formation, egg polarity, and possibly meiosis [21,26]. Based on database analyses, three piwi homologs have been identified in the mouse genome. Two of these genes, Miwi and Mili (Miwi like; Piwil2 - MGI), have been well examined. Miwinull mice do not complete spermatogenesis, but arrest occurs at the beginning of the round spermatid stage [23], significantly downstream of the germline stem cell division stage.
Further studies have revealed the repeat-associated siRNAs (rasiRNAs) in Drosophila [27,28]. The rasiRNAs can be defined as a piRNA or a subset of piRNAs, since they also bind to the PIWI subfamily proteins and their production is performed in a Dicer-independent manner [28-30]. Although the mechanisms of piRNA production require further investigation in mammals, a model for the biogenesis of piRNAs has been recently proposed in Drosophila [28-30]. piRNAs are likely to be derived from either the repeated DNA sequence elements or complex DNA sequence elements [28-30]. piRNA master control loci of DNA are transcribed and exported from the nucleus to the nuage, an amorphous electron-dense cellular material found in germ cells.
The PIWI subfamily Ago protein Argonaute 3 (Ago3) binds to sense-strand transcripts of piRNA to form an Ago3-piRNA complex that guides the slicer-mediated cleavage of target antisense-strand transcripts at an adenine (A): uracyl (U) bp, which generates the antisense piRNA precursors, which are long and single-stranded transcripts with U at the 5' end [28-30]. Aubergine (Aub) and PIWI associate with antisense piRNA precursor to form a complex that is catalyzed by the putative nucleases Squash and Zucchini to produce mature antisense-strand piRNAs [28-30]. By contrast, Aub binds to antisense-strand transcripts of piRNA to form an Aub-piRNA complex. This complex guides the slicer-mediated cleavage of target sense strand transcripts, which generates the sense piRNA precursors with an A at nt 10. The sense piRNA precursors associate with Ago3 and are trimmed to mature sense-strand piRNAs [28-30].
In mammals, the exact mechanism of piRNA action(s) has yet to be elucidated has yet to be illuminated. One suggestion is that piRNAs and PIWI proteins form a complex called PIWI-interacting RNA complex (piRC) that triggers gene silencing, supported by the finding that piRC has been extracted and purified from mammalian testis [17]. piRNAs are known to possess diverse functions, including repression of retrotransposons and post-transcriptional regulation including negative and positive regulations [30-32]. In comparison, miRNAs have no effect on the repression of retrotransposons in germ cells [33], indicating that miRNAs and their biogenesis have a distinct pathway different from piRNAs in suppressing retrotransposons and germ cell development. The roles of piRNAs in spermatogenesis are supported by the known functions of their partner, the PIWI proteins. Several studies have accumulated evidence indicating that the PIWI subfamily proteins, including MIWI, MIWI2, and MILI, are required for stem cell self-renewal and the development of male germ cells in invertebrates [12,18,21,34].
MIWI (PIWIL1)
In detail, a murine piwi gene (miwi) encodes a cytoplasmic protein specifically expressed in spermatocytes and spermatids. Miwinull mice display spermatogenic arrest at the beginning of the round spermatid stage, resembling a phenotype of cAMP-responsive element upmodulator (CREM) a master regulator of spermiogenesis [23]. Furthermore, mRNAs of Act (activator of CREM in testis) and Crem target genes are downregulated in Miwi null testes. Whereas MIWI and CREM do not regulate each other's expression, MIWI complexes with mRNAs of Act and Crem target genes [23]. A human homolog of Piwi and Aub called Hiwi was first isolated from a testis cDNA library, hinting at a potential role for Piwi family genes in mammalian spermatogenesis [21]. The cloning and functional analysis of Miwi as the murine ortholog of Hiwi showed that Miwi is a testis-specific gene encoding a cytoplasmic protein present specifically in spermatocytes and round spermatids [23]. MIWI, a cytoplasmic protein, is first detectable at 14 dpp (days post partum) [23], a stage that corresponds to the appearance of pachytene stage spermatocytes (Figure 1). Consistently, in the adult testis, MIWI is present in midpachytene stage spermatocytes and round spermatids during stages V-VII of the cycle of the seminiferous tubule [35]. It becomes much more abundant in diplotene stage spermatocytes during stage XI of the cycle [23]. MIWI is a key regulator of spermiogenesis, causing Miwi null mice to be arrested at early spermiogenesis [23].
MILI (PIWIL2)
Although Mili and Miwi are expressed in germ cells, their expression kinetics are different [36]. According to the kinetics of expression, the stages of spermatogenetic arrest differ between the Mili-/- and Miwi-/- testes. Arrest was observed at the early pachytene spermatocyte and round spermatid stages in Mili-/- and Miwi-/- testes, respectively [37]. In other words, the expression of Mili was detected up to the pachytene spermatocytes, whereas that of Miwi was detected from the mid-pachytene stage to the emergence of elongated spermatids [36]. Thus, Mili is expressed at an earlier stage than Miwi, and the expression of Mili overlaps somewhat with that of Miwi in the mid pachytene stage (Figure 1).
MILI, in brief, is central to the primary processing of sense piRNAs from retrotransposons and other cellular transcripts [31,37,38]. Then, the primary piRNAs guide the production of secondary piRNAs, which are loaded onto MIWI2, from mostly antisense RNAs transcribed from retrotransposons and other genome elements [37]. The loss of Mili leads to a gross reduction in total piRNAs and those loaded onto MIWI2, while Miwi2-/- mutation affects a more limited number of piRNAs [31,37,38].
MIWI2 (PIWIL4)
The expression of piRNAs is reduced under Mili- and Miwi2-null conditions in fetal germ cells, although the extent of the reduction differs significantly between the two mutants [38]. Both Mili- and Miwi2-targeted mice are sterile because of impaired spermatogenesis at the pachytene stage [38]. Miwi2-deficient mice display a meiotic-progression defect in the early prophase of meiosis I and a marked and progressive loss of germ cells with age [32]. As is seen in other organisms, the expression of the three murine piwi proteins, MIWI (PIWIL1), MILI (PIWIL2), and MIWI2 (PIWIL4), is largely germline restricted [36,39]. Whereas MILI and MIWI are cytoplasmic proteins, MIWI2 is found in the nucleus and is expressed only for a short time during development (Figure 1). It is interesting to note that MIWI2 is found in the cytoplasm in MILI knockout mice [37]. However, in Miwi2 knockout mice, MILI is still localized in the cytoplasm, indicating that MILI is able to direct the nuclear localization of MIWI2 [37].
Thus far, MIWI and MILI have been characterized in some detail, with mice bearing targeted mutations in either Miwi [23] or Mili [37] being male sterile. Although both MIWI and MILI are involved in regulation of spermatogenesis, loss of either protein produces distinct defects that are thematically different from those seen upon mutation of Drosophila Piwi. Based upon their expression patterns and the reported phenotypes of mutants lacking each protein, the most parsimonious model is that both MIWI and MILI perform roles essential for the meiotic process. A recent study indicates that the sterility observed in female flies bearing mutations in PIWI-family proteins is also likely to result, at least in part, from the deleterious effects of transposon activation [29].
Action of PIWI proteins
MOV10L1, a germ cell-specific putative RNA helicase that contains all of the conserved helicase motifs, including ATPase and unwindase domains, is associated with PIWI proteins and abundantly associated with the MILI-piRNA complex, and, to a lesser extent, with the MIWI-piRNA complex in adult testis and also takes a central role in the biogenesis and/or stability of MILI-, MIWI2-, and possibly MIWI-bound piRNAs [40]. Members of the RNA helicase superfamily are required for all biological processes involving RNA molecules, such as ribosome biogenesis, splicing, translation, and RNA interference [41]. A study finding that the truncated mutant protein lacks only the RNA helicase domain and is defective in piRNA biogenesis demonstrates that this domain is required for MOV10L1 functions [40]. Biochemical studies of Drosophila Armi suggest that Armi facilitates ATP-dependent incorporation of single-stranded siRNA into RISC [42]. In embryonic germ cells, MILI and MIWI2 are associated with repeat-rich prenatal piRNAs [37,38]. Taken together with their findings in P10 and P14 mutant testes, MOV10L1 is required for biogenesis and/or stability of both perinatal (MILI- or MIWI2-bound) and prepachytene (MILI-bound) piRNAs [40].
Tudor-domain containing 9 (Tdrd9) is essential for silencing Line-1 retrotransposon in the male mouse germline [43]. Tdrd9 encodes an ATPase/DExH-type helicase and is shown to be essential for male meiosis and retrotransposon silencing in mice; its mutation causes male sterility, revealing meiotic failure [43]. Previous studies have shown that MILI and MIWI2, two mouse PIWI proteins, are central to the feed-forward or ping-pong processing of piRNAs from retrotransposons and other cellular transcripts, and this small RNA pathway is essential for retrotransposon control and DNA methylation in the germline [31,32,37,38]. TDRD9 is also demonstrated to act as a functional partner of MIWI2 in the piwi pathway [32,33], and, furthermore, the tudor-PIWI interaction is proposed to be a key conserved feature in the germline that regulates retrotransposons at the RNA and epigenetic levels to safeguard genetic information [43].
In Tdrd9-/- mutants, Line-1-derived piRNA sequences were significantly reduced or undetectable in the antisense orientation in fetal prospermatogonia, suggesting that TDRD9 participates in proper biogenesis of secondary piRNAs, which are associated with MIWI2, with a preference for Line-1. The loss of Tdrd1 also affects secondary antisense piRNAs, with an increase in nonrepetitive piRNAs loaded onto MILI [44,45]. These data suggest that a possible function of the TDRD9 and TDRD1 proteins is to ensure proper selection and processing of adaptive piRNA sequences in the feed-forward/ping-pong amplification cycle. The distinct compartments of MIWI2-TDRD9 and MILI-TDRD1 [44-46] indicate that these two tudor-PIWI complexes represent functionally separate assemblies of the small RNA machinery, which cooperatively and interdependently function in piRNA biogenesis and retrotransposon silencing [43]. TDRD9 genetically and physically associates with MIWI2, providing evidence that the tudor-piwi interaction is a conserved and essential feature that is responsible for retrotransposon control in male germ cells [43].
Caenorhabditis elegans contains a large set of argonaute/PIWI-related proteins, including two closely related to PIWI called Piwi-related gene (Prg)-1 and Prg-2 [21,47]. The Prg-1 and Prg-2 genes share 40.1% and 38.5% amino acid identity, respectively, with Piwi over their entire length [21]. Moreover, Prg-1 and Prg-2 are 90% identical to each other over their full length and 98% identical at the carboxyl terminus [21]. The PIWI-like protein PRG-1 is localized to P granules in germ cells entering spermatogenesis and is required for successful spermatogenesis at elevated temperature [21,47]. Prg-1 mutant sperm, hence, exhibit extensive defects in activation and fertilization [47-49]. Unlike Prg-1, Prg-2 failed to be actively involved in fertility [47]. In a recent study, the role of two argonaute (AGO)-class paralogs, Alg-3 (T22B3.2) and Alg-4 (ZK757.3), in promoting thermotolerant male fertility is elucidated. Alg-3/4 mutants produce near wild-type numbers of spermatids; these spermatids exhibit severe defects in the activation process called spermiogenesis that converts spermatids into motile ameboid sperm [47]. Translin is a DNA/RNA-binding protein involved in mRNA transport and translation in postmeiotic male germ cells [47]. The noncoding RNAs, Nct1 and 2, contain sequences identical to piRNAs and appear to be male germ cell-specific transcripts, and are predominantly detected in pachytene spermatocytes [50,51]. Nct1/2-deficient mice display a decrease in a small cluster of piRNAs (e.g., antisense LINE 1-related repeat sequence piRNA) in chromosome 2, but this does not affect mouse spermatogenesis or fertility, suggesting that these piRNAs on chromosome 2 are necessary to maintain transposon silencing [51].
Conclusion
It is clear that PIWI proteins are involved in germline development of many metazoan species and are needed for the biogenesis of piRNAs. In summary, in mammals, MIWI, MIWI2, and MILI proteins are expressed in mid- and late-stage germ cells and are essential for mouse spermatogenesis [23,32,52]. The MIWI protein is present in the cytoplasm of spermatocytes as well as in the chromatoid body and cytoplasm of round spermatids, and more importantly, MIWI associates with piRNAs in translation machinery and mRNA stability [16]. Notably, spermatogenesis is arrested at the pachytene spermatocyte stage in Mili-knockout mice [38] and at the round spermatid stage without elongated spermatids or mature spermatozoa production in Miwi-deficient mice [23], while Miwi2-deficient mice display a defect in the early prophase of meiosis I and a marked and progressive loss of germ cells with age [32]. piRNAs are expressed in male germ cells, particularly pachytene spermatocytes and round spermatids. In addition, the small non-coding RNAs have recently been suggested to be piRNA precursors and they are expressed predominantly in pachytene spermatocytes. Thus, it is likely that piRNAs, the partners of PIWI subfamily proteins, are potentially involved in regulating the processes of meiosis and post-meiosis of male germ cell development. To date, studies on PIWIs signify their importance and their potential role in epigenetic regulation and the initial formation of pole cells in flies through the interaction of the cytoplasmic components of the miRNA machinery. This fascinating new field offers many questions still waiting for answers. Growing evidence shows that piRNAs are involved in spermatogenesis. Further studies are more likely to provide better insights into unresolved aspects of idiopathic male infertility.
Notes
This work was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A080364) and a grant of the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (C00552).
No potential conflict of interest relevant to this article was reported.