Endometrial histology and predictable clinical factors for endometrial disease in women with polycystic ovary syndrome
Article information
Abstract
Objective
This study was aimed to investigate endometrial histology and to find predictable clinical factors for endometrial disease (hyperplasia or cancer) in women with polycystic ovary syndrome (PCOS).
Methods
We investigated the endometrial histology and analyzed the relationship between endometrial histology and clinical parameters, such as LH, FSH, estradiol, testosterone, fasting and 2 hours postprandial glucose and insulin, insulin resistance, body mass index, endometrial thickness, menstrual status from 117 women with PCOS. Statistical analysis was performed with chi square and t-test, p-value<0.05 was considered as statistically significant. And receiver operating characteristic curve was used to find predictable clinical factors for endometrial disease and to decide the cuff off values.
Results
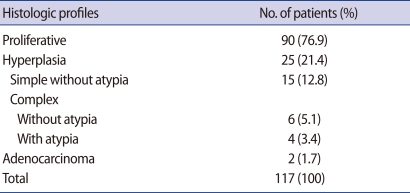
In 117 women with PCOS, endometrial histologic profiles are as follows: proliferative phase in 90 women (76.9%), endometrial hyperplasia in 25 women (21.4%), and endometrial cancer in 2 women (1.7%). Of 25 women with endometrial hyperplasia, simple hyperplasia without atypia, complex hyperplasia without atypia and complex hyperplasia with atypia were diagnosed in 15 (12.8%), 6 (5.1%), 4 (3.4%) women, respectively. Age and endometrial thickness were significantly related with endometrial disease, p=0.013 and p=0.001, respectively. At the cut off level of 25.5 years in age, sensitivity and specificity predicting for endometrial disease were 70.4% and 55.6%, respectively (p=0.023). At the cut off level of 8.5 mm in endometrial thickness, sensitivity and specificity were 77.8% and 56.7%, respectively (p=0.000).
Conclusion
In women with PCOS, the incidence of endometrial hyperplasia and cancer were 21.4% and 1.7%. The age and endometrial thickness may be used as clinical determining factors for endometrial biopsy.
Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrine-metabolic disorder in women of reproductive age with 5-10% of prevalence [1]. An international consensus group proposed that at least two out of three criteria have to be met in order to fit the diagnosis of PCOS. These criteria are oligo-anovulation (usually manifested as oligomenorrhea or amenorrhea), elevated levels of circulating androgens (hyperandrogenemia) or clinical manifestation of androgen excess (hyperandrogenism), and polycystic ovaries as defined by ultrasonography. Also the syndrome can be diagnosed after the exclusion of other known disorders, such as congenital adrenal hyperplasia, Cushing syndrome, and androgen producing tumors [2]. There are increasing evidences that the endocrinologic and metabolic abnormalities in PCOS may have complex effects on the endometrium, contributing to the endometrial disorders observed in women with this syndrome [3]. In women with PCOS, the endometrium is exposed to the prolonged stimulatory effects of unopposed estrogen by chronic anovulation. And as the expression of androgen receptor in the endometrium is up-regulated by estrogens and inhibited by progesterone, in women with PCOS elevated endometrial expression of the androgen receptor has been documented [4]. In addition, because endometrium is associated with insulin-like growth factor-1 (IGF-1) activation, decreased concentrations of sex hormone-binding globulin (SHBG) and upregulation of endometrial aromatase, hyperandrogenemia and hyperinsulinemia may further increase the potential for neoplastic change in the endometrium [3,5,6]. Insulin resistance and hyperinsulinemia consequent to obesity may trigger or unmask the syndrome in genetically predisposed individuals. Therefore, in PCOS with prolonged unopposed estrogen, hyperinsulinemia, elevated free IGF-1 and androgens may further augment the mitogenic activity of endometrial cells, leading to acceleration of hyperplasia and perhaps transformation to cancer [7,8].
However, it is still unclear whether all PCOS patients require an endometrial biopsy to exclude endometrial disease and there are predictable clinical factors for the endometrial disease.
So, we performed this study to investigate the endometrial histology and find the predictable clinical factors for the endometrial disease in women with PCOS.
Methods
This prospective study included 117 consecutive women with PCOS, defined as the 2003 Rotterdam criteria (2 out of 3): 1) oligo-anovulation (menstrual cycle>37 day), 2) clinical or biochemical hyperandrogenism (total testosterone>2 SD), 3) ultrasound feature of polycystic ovaries with 12 or more follicles of 2-9 mm diameter in each ovary. Clinical variables such as age, body weight and height were assessed in all subjects during a visit in the outpatient department. Body mass index (BMI) was calculated as weight (kg) divided by the square of height (m2). A blood hormone test for LH, FSH, as well as E2 and testosterone (T), was sampled on day 3 of the natural or induced menstrual cycle in PCOS patients. Blood hormone levels were assessed using commercial kits, as follows; LH, FSH by immunoradiometric assay (IRMA; DIAsource SA, Nivelles, Belgium), E2 by radioimmunoassay (RIA; DIAsource SA) and serum total T by RIA (Radim SpA, Rome, Italy). Fasting and postprandial 2 hours glucose and insulin levels were evaluated by a 75 g oral glucose tolerance test using commercial kits (Immunotech, Prague, Czech Republic) in order to assess insulin resistance in all patients. The endometrial thickness was measured by vaginal or rectal route, as the widest distance from the reflective interface between the endometrium and myometrium of one side to the opposing side on a sagittal plane of the uterus [9], using a 5-MHz linear-array vaginal transducer (SSD 1000; Aloka, Tokyo, Japan). Because there were no clear clinical guidelines to suggest when an endometrial biopsy should be done [8], the endometrial biopsy was performed in the all participants. All vaginal or rectal ultrasound assessments and endometrial biopsies were performed by one investigator to preclude the interobserver variation. The endometrial tissues were sent for pathological diagnosis and we investigated the incidence of each histologic pattern. Two main histologic types were classified as proliferative endometrium and diseased endometrium. Simple, complex hyperplasia with or without cytologic atypia, or adenocarcinoma were included within the diseased endometrium because of the relatively small number in each of the hyperplastic subcategories or adenocarcinoma. Then we compared clinical parameters, such as LH, FSH, E2, testosterone, fasting and 2 hours postprandial glucose and insulin, insulin resistance, BMI, age, endometrial thickness and menstrual status between 2 groups to find the predictable clinical factor for endometrial disease in women with PCOS. In addition, a subgroup analysis was followed in some clinical parameters such as menstrual pattern (amenorrhea or oligomenorrhea), 2 hours gluocose (<140, 140-200, >200), 2 hours insulin (100-150, 151-300, >300) and fasting glucose/insulin (<4.5, ≥4.5).
Clinical parameters between 2 groups were compared using a chi-square test and the Student's t-test. And receiver operating characteristic (ROC) curve analysis was used to find the predictable clinical factor for endometrial disease and decide the cut off values. A p-value<0.05 was considered as statistically significant.
Results
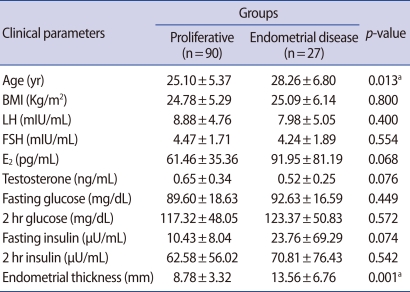
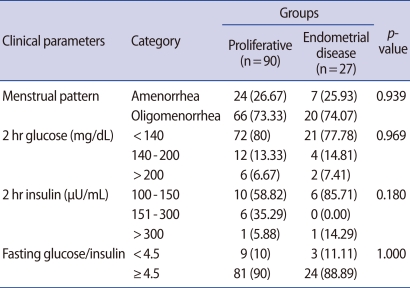
In total of 117 PCOS patients, proliferative endometrium was found in 90 women with PCOS (76.9%), endometrial hyperplasia was diagnosed in 25 cases (21.4%) and endometrial adenocarcinoma was diagnosed in 2 cases (1.7%). Of the 25 cases of endometrial hyperplasia, 15 (12.8%), 6 (5.1%), and 4 (3.4%) cases were classified as simple hyperplasia without cytologic atypia, complex hyperplasia without, and with atypia, respectively (Table 1). Two main histologic types were proliferative endometrium and endometrial disease. Of a all clinical parameters (age, BMI, LH, FSH, E2, testosterone, fasting glucose, 2 hours postprandial glucose, fasting insulin, 2 hours postprandial insulin, endometrial thickness), the mean age of the women with endometrial disease was significantly higher than that of women with proliferative endometrium (28.26±6.80 years vs. 25.10±5.37 years; p=0.013). The mean thickness of the endometrium was significantly thicker in endometrial disease group, compared with the proliferative endometrium group (13.56±6.76 mm vs. 8.78±3.32 mm; p=0.001). There were no significant differences in the remained clinical parameters listed. The values of clinical parameters between 2 endometrial histologic groups are summarized in Table 2. In the subgroup analysis of the menstrual pattern, 2 hours glucose and insulin, and fasting glucose/insulin ratio, there was no statistically significant difference between 2 groups (Table 3). Only the endometrial thickness and age were found to be significant predictors of endometrial disease in women with PCOS.
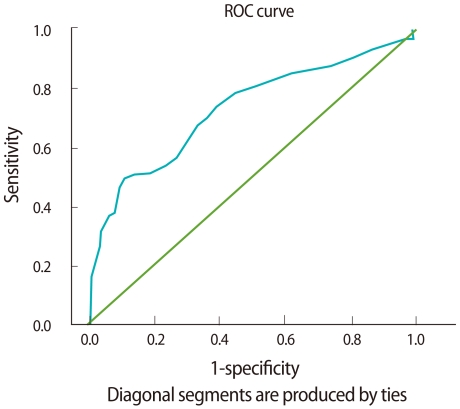
The endometrial thickness and age were further evaluated in the ROC curve analysis. The areas under the ROC curves for age and endometrial thickness were both greater than 0.5 (Figures 1, 2). The areas under the ROC curves for age and endometrial thickness were, respectively, 0.645 (95% confidence interval [CI], 0.518-0.771; p<0.05) and 0.734 (95% CI, 0.615-0.852; p<0.01). Also, using an endometrial thickness of 8.5 mm as the cut off level predicting for endometrial disease, the sensitivity and specificity would be 77.8% and 56.7%, respectively; using an age of 25.5 years as the cut off level, the corresponding values would be 70.4% and 55.6%.

Receiver operating characteristic (ROC) curve for age. The closer the ROC plot is to the upper left corner, the higher the overall accuracy of the test. When the variable under study cannot distinguish between the two groups, the area under the ROC curve is equal to 0.5 (diagonal straight line).
Discussion
PCOS is a complex disorder with metabolic and endocrine manifestations and seems to have long term health consequences of the syndrome [10]. In addition to the diabetes, cardiovascular disease and breast cancer, the effect of the unopposed and uninterrupted estrogenic stimulation is to place the patient at considerable risk for endometrial cancer [5,10,11]. The known factors which increase the risk of developing endometrial hyperplasia and cancer are obesity, nulliparity, infertility, hypertension and diabetes [12]. Most of these factors are also known to be associated with PCOS [10]. Therefore, it is important to evaluate the endometrium and recognize the clinical factors that are likely to have affected on endometrial disease in women with PCOS. To our knowledge, this is likely to be the first study to investigate the endometrial histology and find the predictable clinical factors for endometrial disease in Korean women with PCOS. The incidence of endometrial disease in our study was 23.1% of women with PCOS and of these, 21.4% and 1.7% were endometrial hyperplasia and endometrial cancer, respectively. In another study [8], the endometrial hyperplasia was diagnosed in 35.7% of women with PCOS who were not receiving either contraceptive steroids or periodic or monthly progestin withdrawal. In a long-term follow up study of women with PCOS based on ovarian wedge resection, the odds ratio for endometrial cancer was 5.3 (95% CI, 1.55-18.60) compared with control subjects [12]. In a larger study of 1,270 women with chronic anovulation, the relative risk of endometrial cancer was identified to be 3.1 (95% CI, 1.1-7.3) and the risk was only significantly increased in the subgroup of overweight women [13]. Then, the question whether all PCOS patients require an endometrial biopsy to exclude endometrial disease remains unanswered and if so, predictable clinical factors for endometrial disease are questionable. Many authors agree that in postmenopausal women with uterine bleeding, the endometrial thickness on vaginal ultrasound assessment has been positively correlated with the presence of endometrial abnormalities, and curettage can be avoided if the endometrial thickness is less than 4 mm [14,15]. In PCOS patients, unfortunately there are no clear clinical guidelines to determine when the endometrial biopsy should be performed [8]. Cheung [8] demonstrated that in anovulatory, infertile women with PCOS, endometrial thickness greater than 7 mm or intermenstrual interval of more than 3 months can be associated with endometrial hyperplasia and an endometrial biopsy would be recommended. Other authors propose that endometrial biopsy is indicated when the clinical history suggests long-term unopposed estrogen exposure even when the endometrial thickness is normal (5-12 mm), and that biopsy should be performed when endometrial thickness is greater than 12 mm even when clinical suspicion of disease is low [6]. In our study, although this study has a limitation that has been not shown an intermenstrual interval or the duration of exposure to unopposed estrogen, the endometrial thickness and age were positively correlated with the presence of endometrial disease in women with PCOS. Also, our study showed that in women with PCOS, endometrial thickness more than 8.5 mm or age more than 25.5 years could be associated with the endometrial disease.
In conclusion, it seems that the endometrium in women with PCOS is different from it of healthy women in the endocrinological aspect, and is consistent with a higher incidence of hyperplasia and cancer [16]. Theoretically, there are many clinical factors developing endometrial hyperplasia or cancer, but an endometrial biopsy to exclude endometrial disease should be considered according to the clinical status. And also, the results of this study suggest that the age and endometrial thickness may be used as clinical determining factor for endometrial biopsy.
Notes
No potential conflict of interest relevant to this article was reported.