Effects of various combinations of cryoprotectants and cooling speed on the survival and further development of mouse oocytes after vitrification
Article information
Abstract
Objective
The objectives of this study were to analyze efficacy of immature and mature mouse oocytes after vitrification and warming by applying various combinations of cryoprotectants (CPAs) and/or super-rapid cooling using slush nitrogen (SN2).
Methods
Four-week old ICR female mice were superovulated for GV- and MII-stage oocytes. Experimental groups were divided into two groups. Ethylene glycol (EG) only group: pre-equilibrated with 1.5 M EG for 2.5 minutes and then equilibrated with 5.5 M EG and 1.0 M sucrose for 20 seconds. EG+dimethylsulfoxide (DMSO) group: pre-equilibrated with 1.3 M EG+1.1 M DMSO for 2.5 minutes and equilibrated with 2.7 M EG+2.1 M DMSO+0.5 M sucrose for 20 seconds. The oocytes were loaded onto grids and plunged into SN2 or liquid nitrogen (LN2). Stored oocytes were warmed by a five-step method, and then their survival, maturation, cleavage, and developmental rates were observed.
Results
The EG only and EG+DMSO groups showed no significant difference in survival of immature oocytes vitrified after warming. However, maturation and cleavage rates after conventional insemination were greater in the EG only group than in the EG+DMSO group. In mature oocytes, survival, cleavage, and blastocyst formation rates after warming showed no significant difference when EG only or EG+DMSO was applied. Furthermore, cleavage and blastocyst formation rates of MII oocytes vitrified using SN2 were increased in both the EG only and EG+DMSO groups.
Conclusion
A combination of CPAs in oocyte cryopreservation could be formulated according to the oocyte stage. In addition, SN2 may improve the efficiency of vitrification by reducing cryoinjury.
Introduction
Since the first successful pregnancy derived from cryopreserved human oocytes was reported in 1986 [1,2], various freezing and thawing protocols have been applied to the cryostorage of oocytes. Oocyte cryopreservation boasts various advantages over embryo cryopreservation. Oocyte cryopreservation would significantly contribute in assisted reproductive technology (ART) programs. It allows patients to cryopreserve their oocytes when they have no partner or are about to lose their ovarian function due to surgery, chemotherapy, or radiotherapy or want to delay the delivery [3]. Also, it avoids ethical issues and legal restrictions. Unfortunately, despite remarkable progress, oocyte cryopreservation remains a demanding task, as there is no precise standardization of cryopreservation and warming procedures. Hence, the technique is not yet widely used in clinical practice.
The slow cooling method was initially used for oocyte cryopreservation. However, only a few studies have demonstrated successful outcomes of oocytes that were frozen using a slow cooling method [1,2]. This can be explained by zona pellucida hardening from premature cortical granule exocytosis, chromosomal nondisjunction caused by serious disturbance of the microtubules, disturbance in pronuclear (PN) formation, and polar body release from microfilament damage and cytoskeleton alteration after cryopreservation [4-10]. Several studies have reported effective outcomes of applying various improved systems including reducing the concentration of sodium in cryoprotectants (CPAs) [11,12] or using different types and concentration of CPAs during cryopreservation [13-17]. In addition, some studies reported excellent survival, embryonic development, and pregnancy rates by applying a vitrification method which avoids the formation of ice crystals using a high concentration of CPA and ultra-rapid cooling speed [18]. Advantages of vitrification include that it is time-saving, easy to perform, and does not require expensive equipment. Most importantly, it could minimize the damage to oocytes in that it avoids the forming of ice crystals. However, its drawback is that it requires a high concentration of CPA, which may cause toxicity and osmotic damage to the oocyte. Since the development of vitrification, the toxicity of CPA has been the one of the major concerns in applying this method as a cryopreservation method. Several studies have been conducted to avoid the drawback by using CPA with high speed permeability and less toxicity or by applying a combination of permeable and non permeable CPAs to decrease the absolute concentration without a decrease in the relative concentration. Also, in order to increase the cooling rate for vitrification, many studies have been attempted for minimizing the CPA solution volume or improving the heat conductivity of cryo-equipment. Furthermore, using liquid nitrogen in a slush state (slush nitrogen, SN2) has improved the survival and embryonic development rates after vitrification of oocytes and embryos and this may be due to the increase in the heat transfer rate that is associated with SN2 [19,20].
Ethylene glycol (EG) is widely used in the vitrification method as it is one of the major permeable CPAs with a low molecular weight, and it is also less toxic to mammal oocytes or embryos including humans [21-24]. In particular, the strategy for reducing cell injury by applying a short exposure to a high concentration of CPA has been widely used. In contrast, Mukaida et al. [25] has recently reported a high survival rate of embryos that were vitrified at a blastocyst stage with a combination of EG and dimethylsulfoxide (DMSO, a slow permeable CPA). The combination of CPAs for the vitrification process may have induced a lower relative concentration and also lower toxicity of CPAs. In fact, as DMSO penetrates into the cell, it accelerates its characteristics of glass-forming and it increases the permeability rate as it combines with the other types of CPAs which complement each other. Also, there has been a report that when a combination of CPAs was used, a higher blastocyst development was obtained compared to using only 40% EG in bovine oocytes [26]. These studies have been conducted only with embryos or blastocyst stage embryos, but not many studies have worked with germinal vesicle (GV) stage or metaphase II (MII) stage oocytes. In addition, few studies have been conducted regarding the cooling rate, which is one of factors that affect oocytes or embryos during vitrification [27]. Therefore, in this study, we analyzed the survival and subsequent embryonic development rates of immature and mature mouse oocytes after a vitrification and warming process using combinations of CPAs and/or using SN2 to develop an efficient vitrification method for immature and mature oocytes.
Methods
1. Preparation of immature and mature oocytes
ICR mice (Samtako, Seoul, Korea) were maintained in a temperature-and-humidity-controlled room under a 12 hours: 12 hours light: dark cycle. For immature oocyte collection, four-week-old female mice were superovulated via an intraperitoneal injection of 5 IU pregnant mare serum gonadotropin (PMSG; Dae Sung Microbiological Labs, Seoul, Korea). At 44-46 hours post PMSG, mice were sacrificed by cervical dislocation for the collection of ovaries. The ovaries were then transferred to Quinn's advantage medium with HEPES (Quinn's-HEPES; Sage, In Vitro Fertilization, Trumbull, CT, USA) containing 10% substitute protein serum (SPS; Sage BioPharma, Inc., Bedminster, NJ, USA). Immature oocytes were collected by puncturing of follicles with a needle (29 G). Cumulus-enclosed immature oocytes were selected for the experiment. For the collection of mature oocytes, 4-week-old ICR female mice were superovulated with 5 IU PMSG, followed by injection with 5 IU human chorionic gonadotropin after 48 hours (hCG; Intervet, Boxmeer, the Netherland). Cumulus-enclosed mature oocytes were retrieved at 13.5-14 hours post-hCG from the oviducts.
2. Vitrification and warming of oocytes
Quinn's-HEPES with 20% (v/v) fetal bovine serum (FBS; Gibco, Grand Island, NY, USA) was used as the base medium for preparation of all vitrification and warming solutions. As a CPA, EG (Sigma-Aldrich, St. Louis, MO, USA) only or a combination of EG and DMSO (Sigma-Aldrich) were used for the vitrification procedure. For group 1, oocytes were pre-equilibrated with 1.5 M EG for 2.5 minutes, followed by equilibration with 5.5 M EG and 1.0 M sucrose for 20 seconds. For group 2, oocytes were pre-equilibrated with 1.3 M (7.5%) EG and 1.1 M (7.5%) DMSO for 2.5 minutes, followed by 2.7 M (15%) EG, 2.1 M (15%) DMSO and 0.5 M sucrose for 20 seconds. CPA-equilibrated oocytes were loaded onto an electron microscopic (EM) copper grid and plunged into liquid nitrogen (LN2) or SN2. SN2 was produced using a Vit-master (IMT, Ness Ziona, Israel). The concentration of CPA was determined according to the study of Mukaida et al. [25]. Vitrified immature and mature oocytes were stored for at least 2 weeks and then warmed to compare their survival and subsequent embryonic development.
The vitrified oocytes were warmed by a five-step method. The copper grids were sequentially transferred to 1.0, 0.5, 0.25, 0.125, and 0 M sucrose with an interval of 2.5 minutes. The vitrified/warmed oocytes were then washed with fresh culture medium for three times. The immature oocytes were transferred to the culture medium and observed under a microscope for the survival rate of oocytes 1 hour after warming. The surviving immature oocytes were then induced to develop into mature oocytes by culturing them in the in vitro maturation medium. The mature oocytes from the immature stage and the survived mature oocytes obtained earlier were fertilized in vitro for further experimentation.
3. In vitro maturation of oocytes
Survived immature oocytes from the vitrification/warming process were induced for maturation in G2.3 medium (Vitrolife, Göteborg, Sweden) containing 20% FBS, 0.075 IU/mL FSH (Gonal-F, Serono, Modugno Bari, Italy), 0.5 IU/mL hCG (Ovidrel, Serono) and 1.0 µg/mL estradiol (Sigma-Aldrich) for 14 hours. Cumulus cells from mature oocytes were removed with modified Dulbecco's phosphate-buffered saline (D-PBS, HyClone, South Logan, UT, USA) solution containing 0.1% hyalurodase (Sigma-Aldrich). Denuded oocytes were washed with fresh culture medium three times and their maturation rate was then evaluated by observation of the first polar body under the microscope.
4. In vitro fertilization and culture
Epididymal spermatozoa were obtained from 8- to 10-week-old male ICR mice. Sperm suspension was capacitated in the incubator at 37℃, in 5% CO2 in air for 90 minutes. Capacitated spermatozoa were mixed (1-2×106/mL) with cumulus-oocyte complex in Quinn's advantage fertilization medium and incubated for 6 hours. The oocytes were then washed three times in modified simplex-optimized medium (KSOM; Millipore, Danvers, MA, USA) supplemented with 0.3% bovine serum albumin (BSA; Sigma-Aldrich). The fertilized oocytes were cultured in KSOM under 37℃ in 5% CO2 for 5 days to analyze embryonic development.
5. Statistical analyses
Survival and maturation rates of oocytes, embryonic development, and blastocyst formation rates were analyzed for statistical significance with one-way ANOVA (Duncan-test). p-values<0.05 were considered statistically significant.
Results
1. Effect of CPA on the survival, maturation, and embryonic development of immature oocytes after vitrification using LN2 or SN2
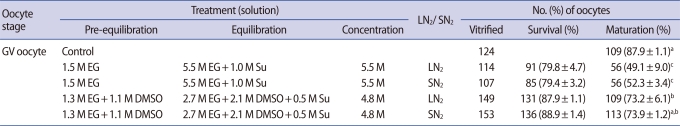
The survival and maturation rates of immature oocytes were compared after the vitrification/warming process using EG only or a combination of EG+DMSO as CPAs (Table 1). After vitrification/warming using LN2, there was no significant difference in the survival rates of the EG only group and the EG+DMSO group (79.8±4.7% vs. 87.9±1.1%; p>0.05), nor was there any difference between the groups when using SN2 (79.4±3.2% vs. 88.9±1.4%; p>0.05). However, the EG+DMSO group showed a higher maturation rate than the EG only group in both LN2 and SN2 (73.2±6.1%, 73.9±1.2% vs. 49.1±9.0%, 52.3±3.4 %; p<0.05). The cleave rate of immature oocytes in the EG only group was significantly lower than that of the control and EG+DMSO groups after vitrification using LN2 (26.3±7.8% vs. 62.9±12.2% and 57.7±5.0%; p<0.05). After vitrification using SN2, the cleave rate in the EG group was lower than in the EG+DMSO group, but the difference was not statistically significant (37.4±4.8% vs. 58.8±3.7%; p>0.05). All groups showed a very low blastocyst formation rate. Numerically, a higher rate of blastocyst formation was observed in the EG+ DMSO group after vitrification using SN2, than the other groups but it was not statistically significant (3.9±1.7% vs. 0±0.0%, 1.3±0.7%, 1.9±1.2%; p>0.05).
2. Effect of CPA on the survival, maturation, and embryonic development of mature oocytes after vitrification using LN2 or SN2
Mature oocytes were vitrified and warmed as immature oocytes and the survival rate was evaluated (Table 2). After vitrification using LN2 or SN2, there was no significant difference between the survival rates of the EG only and EG+DMSO groups (71.0±2.4%, 75.0±3.5% vs. 60.0±8.8%, 79.0±4.3%; p>0.05). There were no significant differences in cleave rates in the EG only and EG+DMSO groups after vitrification using LN2 or SN2 (49.0±5.9% vs. 43.0±4.5%, 59.0±1.1% vs. 62.0±2.6%; p>0.05). However, the cleave rate was significantly higher in SN2 than in LN2 in the EG only and EG+DMSO groups (59.0±1.1%, 62.0±2.3% vs. 49.0±5.9%, 43.0±4.5%; p<0.05). Furthermore, a higher blastocyst formation rate was observed in SN2 than in LN2 for the EG+DMSO group. (21.0±2.2% vs. 7.0±0.8%; p<0.05).
Discussion
Cryopreservation of immature oocytes at the GV stage was considered an alternative to avoid the drawbacks of cryopreservation of mature oocytes such as chromosomal nondisjunction induced by damage to the spindle and premature cortical granule exocytosis [28,29]. However, as cumulus cells and cytoplasm are tightly connected together, immature oocyte cryopreservation resulted in major damage and also a low maturation rate; hence, its clinical applications have been limited. Recently, the vitrification process was applied to overcome these drawbacks. In fact, in this study, a high survival rate was obtained by applying vitrification; in particular, using combinations of CPAs, EG, and DMSO in vitrification showed an approximately 90% survival rate (Table 1). The fertilization and cleave rates of oocytes were also higher in the CPA combination group.
In many studies, the pre-equilibrium and equilibrium time were longer when DMSO was used for cryopreservation, as it has a lower permeability rate than EG [30]. Hence, we have conducted a preliminary study and compared the blastocyst formation rate of oocytes and embryos by using different pre-equilibrium and equilibrium times. As a result, there was no difference in the blastocyst formation for various time and, in fact, when oocytes or embryos were treated with DMSO with the same exposure time as to EG, they showed even better results [31]. Consequently, unlike other studies, we used the same exposure time for the EG group and EG+DMSO combination group. Slush nitrogen was used to analyze the effect of the cooling rate. The results showed that there was no particular effect on survival, maturation, or cleavage rates after warming immature oocytes (Figure 1). The fertilization rate showing two PN could not be identified because the conventional insemination method was used in this study and also the blastocyst formation rate was very low. These results may be due to the inefficiency of the vitrification/warming process or the possibility of side effects such as parthenogenesis. Hence, this results in a limitation of the study. For evaluation of the effectiveness of immature oocyte cryopreservation, it is necessary to carry out further research using a method such as intracytoplasmic sperm injection, which directly injects sperm into an oocyte. Furthermore, in this study, only the survival and embryonic development rates were observed, but not intracellular changes that may occur during the vitrification/warming process, and so the direct effect of using a combination of CPAs was not possible to evaluate. Hence, further study is required.

The effect of different cryoprotectants and cooling speed generated by using LN2 or SN2 on the first cleavage (A) and blastocyst formation (B) of immature oocytes following vitrification. a,b,cDifferent superscripts indicate significant differences (p<0.05). LN2, liquid nitrogen; SN2, slush nitrogen; EG, ethylene glycol; DMSO, dimethylsulphoxide.
In mature oocytes, the results obtained were different from those of immature oocytes. After the vitrification/warming process, the survival, cleavage, and blastocyst rates of the EG only and EG+DMSO groups were not significantly different (Table 2, Figure 2). However, when SN2 was used for vitrification, there was no significant difference in the survival rate, but it did increase the cleavage and blastocyst formation rates. Also, the blastocyst formation rate was significantly higher when a combination of CPAs, EG and DMSO, and SN2 were used. As stated previously, this result is may be due to the increase in the efficiency of vitrification by using DMSO, which accelerates its glass-forming characteristics as it penetrates into the cell and also due to SN2, which increases the cooling rate [19,20,25].

The effect of different cryoprotectants and cooling speed generated by using LN2 or SN2 on the first cleavage (A) and blastocyst formation (B) of mature oocytes following vitrification. a,b,cDifferent superscripts indicate significant differences (p<0.05). LN2, liquid nitrogen; SN2, slush nitrogen; EG, ethylene glycol; DMSO, dimethylsulphoxide.
From this study, we observed that adjusting an existing vitrification solution that is generalized worldwide is required in order to increase the efficiency of vitrification, as the characteristics of immature and mature oocytes are different. Also, although applying SN2 in vitrification process did not affect immature oocytes, it did affect the embryonic development of mature oocytes after the warming process. These results are due to the characteristics of mature oocytes which include an exposed spindle that is essential for chromosomal division; however, further study is required to evaluate the mechanism in more detail. In this study, although the survival rate of immature oocytes was as high as that obtained from mature oocytes, immature oocytes still showed a low embryonic development rate after vitrification/warming process. This result was similar to studies of human oocytes [32,33]. It is suggested that this is not only caused by problems with the oocyte cryopreservation process but also the lack of research on the in vitro maturation process. Therefore, if studies on the process of in vitro maturation of oocytes and development of a culture system move forward then the efficiency of immature oocyte cryopreservation should be expected to improve.
In conclusion, the combination of CPAs, EG, and DMSO showed greater effectiveness in the vitrification of immature oocytes while for that of mature oocytes, it is possible to use either EG only or EG and DMSO. In addition, SN2 may improve efficiency by reducing cryoinjury during vitrification.
Notes
This work was partly supported by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A084923) and a grant (2009-0093821) from the Priority Research Centers Program funded by the Ministry of Education, Science and Technology, Republic of Korea.
No potential conflict of interest relevant to this article was reported.