Differentiation of human male germ cells from Wharton's jelly-derived mesenchymal stem cells
Article information
Abstract
Objective
Recapitulation of the spermatogenesis process in vitro is a tool for studying the biology of germ cells, and may lead to promising therapeutic strategies in the future. In this study, we attempted to transdifferentiate Wharton's jelly-derived mesenchymal stem cells (WJ-MSCs) into male germ cells using all-trans retinoic acid and Sertoli cell-conditioned medium.
Methods
Human WJ-MSCs were propagated by the explant culture method, and cells at the second passage were induced with differentiation medium containing all-trans retinoic acid for 2 weeks. Putative germ cells were cultured with Sertoli cell-conditioned medium at 36℃ for 3 more weeks.
Results
The gene expression profile was consistent with the stage-specific development of germ cells. The expression of Oct4 and Plzf (early germ cell markers) was diminished, while Stra8 (a premeiotic marker), Scp3 (a meiotic marker), and Acr and Prm1 (postmeiotic markers) were upregulated during the induction period. In morphological studies, approximately 5% of the cells were secondary spermatocytes that had completed two stages of acrosome formation (the Golgi phase and the cap phase). A few spermatid-like cells that had undergone the initial stage of tail formation were also noted.
Conclusion
Human WJ-MSCs can be transdifferentiated into more advanced stages of germ cells by a simple two-step induction protocol using retinoic acid and Sertoli cell-conditioned medium.
Introduction
Spermatogenesis is a unique, complex, and tightly coordinated process that takes place in the seminiferous tubules. It is broadly divided into three distinct phases: proliferation of spermatogonial stem cells (mitosis), reduction of chromosome number (meiosis), and transformation of spermatids into sperm (spermiogenesis) [1]. Thousands of genes and proteins are involved and sequentially expressed at different stages of spermatogenesis [23]. Effective models for recapitulating this orchestrated process in vitro would facilitate a deeper understanding of germ cell biology, stage-specific disorders, and the onset of some inherited diseases. This knowledge would be useful for possible interventional therapies for selected cases of infertility, genetic manipulations of germ cells, and fertility preservation [45]. Various cell types, such as spermatogonial stem cells (SSCs), embryonic stem cells (ESCs) [6], induced pluripotent stem cells (iPSCs) [7], fibroblasts, myoblasts, mesenchymal stem cells (MSCs) [89], and very small embryonic-like stem cells [10], have been used as initial source for differentiating germ cells. The potential capacity of somatic stem cells to transdifferentiate into primordial germ cells (PGCs), SSCs [111213], or advanced spermatids through meiosis under appropriate culture conditions has been described [1415]. Different culture systems, such as simple organ cultures, refined monolayer cultures, and embryoid body cultures have been employed, using gene-transfected stem cells or nontransfected cells directly [21316]. The clinical value of direct differentiation may be greater, as gene transfection carries the risk of genetic imbalances in genetically modifiedcells.
Bone morphogenetic protein 4 (Bmp4) and glial cell line-derived neurotrophic factor are factors known to promote the transdifferentiation of somatic stem cells into PGCs [1517], and effective induction of meiosis is achieved chemically by retinoic acid, follicle-stimulating hormone (FSH), and kit ligand; mechanically by microgravity [1819]; and physiologically by coculturing with Sertoli cells [20]. Spontaneous meiotic reduction in a minor population of cells has also been noted [17]. The efficiency of the specification and survival of germ cells may be enhanced when they are cultured as embryoid bodies and in the presence of Sertoli cells and gonadotropins [2122]. Medium containing glutamine at a temperature at 34℃ fostered the spermatogenesis process in rat germ cells [23].
Transformation of spermatids into sperm has been achieved using two strategies: three-dimensional culture [24] and transplantation into germ cell-depleted testes [25]. Although complete spermiogenesis is possible with SSC-derived spermatids, no reports of somatic stem cell-derived morphologically normal sperm have yet been published [26]. Round spermatid injection has produced live offspring in animal models, but the epigenetic status of these cells is largely unknown. It is important to develop culture conditions mimicking the precise molecular mechanisms that regulate cross-associations between somatic and germ cells to complete the recapitulation of spermatogenesis in vitro.
Although retinoic acid induces the meiotic reduction of chromosomes, it does not help with progression through spermiogenesis. Lack of somatic support, especially Sertoli cells, is one possible explanation for this phenomenon. Nevertheless, Lee et al. [4] could not go beyond the round spermatid state when reaggregated neonatal bovine testicular cells were cultured for 14 weeks. However, premeiotic mouse SSCs cultured with testicular somatic cells in a three-dimensional culture system produced viable sperm in a previous study [2627]. The germ cell development process in mice shares similarities with the corresponding process in humans through a number of conserved mechanisms. However, variations in culture requirements are to be expected as the genetic requirements for human germ cell development are unique [15]. In this study, we attempted to progress retinoic acid-induced meiotic human MSCs through spermiogenesis by culturing them with Sertoli cell-conditioned medium.
Methods
1. Isolation and expansion of human umbilical cord matrix MSCs
Umbilical cords were obtained from full-term babies at the professorial Gynaecology Unit of the North Colombo Teaching Hospital, Ragama. The study was approved by Ethics Committee of the institute, and informed written consent was obtained from each mother (No. FWA00013225). All chemicals except those mentioned specifically were purchased from Sigma-Aldrich (St. Louis, MO, USA). The umbilical cords were cut into pieces that were approximately 2–3 cm long. A few sections from different sites were selected and washed in cold phosphate-buffered saline (PBS) containing high concentrations of antibiotics (200 U/mL penicillin, 200 mg/mL streptomycin, and 0.50 mg/mL amphotericin B) and thoroughly squeezed with curved forceps. Washing was repeated until all blood trapped within the blood vessels was removed. The umbilical cord pieces were transferred into a new sterile Petri dish containing PBS supplemented with 100 U/mL penicillin, 100 mg/mL streptomycin, and 0.25 mg/mL amphotericin B. Amniotic epithelium and cord vessels were removed carefully to visualize the inner mucoid connective tissue, also known as Wharton's jelly (WJ). Isolated WJ was diced into 2- to 4-mm explants, and transferred into 25-cm2 tissue culture flasks containing 2 mL of Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, 100 mg/mL streptomycin, 2 mM L-glutamine, and 0.1 mM β-mercaptoethanol. The culture plates were incubated at 37℃ and 5% CO2 for 7–10 days until the MSCs were outgrown from the periphery of the tissue fragments. The tissue explants were removed and cultures were continued up to 80%–90% confluence with twice-weekly medium changes. At confluence, cells were lifted using trypsin/EDTA (ethylenediaminetetraacetic acid) and subcultured or stored in liquid nitrogen for future use. The MSCs were characterized using the specific markers CD44, CD73, CD90, and CD105.
2. Preparation of Sertoli cell-conditioned medium
Testicular tissue was obtained from a 45-year-old man who underwent an orchidectomy and provided informed consent. A single-cell suspension was obtained by a two-step enzymatic digestion method. Briefly, decapsulated segments of testis tissue were cut into small pieces and digested with collagenase type IV (1 mg/mL) at 37℃ for 20 minutes with shaking at regular intervals. The tissue fragments were allowed to settle at the bottom of the centrifuge tube, and the supernatant containing germ cells and peritubular cells was removed. This step was repeated twice, and a single-cell suspension was obtained by a second enzymatic digestion process with trypsin-EDTA (0.25%) for 10 minutes at 37℃. A monolayer of Sertoli cells was obtained using the differential plating technique. In this method, the cells were suspended in DMEM supplemented with 10% FBS, 2 mM L-glutamine, 100 U/mL penicillin, and 100 mg/mL streptomycin, and cultured in 0.2% gelatin-coated wells for 24 hours at 37℃ in 5% CO2 to attach the Sertoli cells. The nonadherent cells were removed and the culture was continued up to 80% confluence in a T-25 culture flask (Nest Biotechnology, Wuxi, China). For the preparation of Sertoli cell-conditioned medium, confluent cells were washed with PBS and replenished with 5 mL of serum-free DMEM. After 72 hours of incubation, the medium was collected, centrifuged at 3,000 rpm for 10 minutes, filtered using a 0.22-µm filter, and stored at −20℃ until use.
3. Germ cell differentiation from MSCs
Cells at the third passage were grown in 24-well culture plates up to 70% confluence. At this stage, the cells were washed twice with PBS, and introduced into freshly prepared DMEM supplemented with 10% FBS, 100 U/mL penicillin, 100 mg/mL streptomycin, 2 mM L-glutamine, and 10 µM all-trans retinoic acid (ATRA). Induction was continued for 14 days at 36℃ in 5% CO2, refreshing the medium on alternate days. Cells in six wells were used for gene expression studies on day 14. The rest of the cells were introduced into Sertoli cell-conditioned medium, and induced for 3 further weeks. The medium supplemented by FBS was replaced with adult human male serum (10%) in this step. Morphological changes were observed in a trypsinized single-cell suspension at the end of the differentiation period. All procedures were repeated five times using five different cord tissue samples.
4. RNA extraction and reverse-transcription polymerase chain reaction
Total RNA was extracted from the controls and induced MSCs using the RNA Xpress reagent (HiMedia, Pune, India) according to the manufacturer's instructions. The mRNA was reverse-transcribed to cDNA (complementary DNA) using 1 µg of total RNA, 10 µM oligo-dT primers, 1×M-MLV reverse transcriptase, 10 mM DTT, and 5 mM dNTP following standard protocols. The final polymerase chain reaction (PCR) reaction mixture contained 10 ng of cDNA, 1×buffer, 2 mM MgCl2, 200 nM forward primer, 200 nM reverse primer, 200 µM concentrations of each dNTP, and 0.5 U of Taq polymerase. The primer sequences and annealing temperatures were as follows: Oct4: forward, 5′-TGCTCCCTCACTTTGCTTC-3′; reverse, 5′-CTCCAGGTTGCCTCTCACTC-3′, 60℃; Plzf: forward, 5′-CGGTTCCTGGATAGTTTGC-3′; reverse, 5′-GGGTGGTCGCCTGTATGT-3′, 55℃; Stra8: forward, 5′-AGCAGCTTAGAGGAGGTCAAGA-3′; reverse, 5′-TACTCGGAACCTCACTTTTGTC-3′, 52℃; Scp3: forward, 5′-TGCAGAAAGCTGAGGAACAAG-3′; reverse, 5′-CTTGCTGCTGAGTTTCCATCA-3′, 54℃; Acr: forward, 5′-ATGACTGGAGACTGGTTTTCGG-3′; reverse, 5′-CTTAGCACGGGCACAGCCTA-3′, 56℃; Prm1: forward, 5′-CAGAGTTCCACCTGCTCACA-3′; reverse, 5-TTCTCAGGCAGGAGTTTGGT-3′, 60℃; β-actin: forward, 5′-GGACTTCGAGCAAGAGATGG-3′; reverse, 5′-AGCACTGTGTTGGCGTACAG-3′, 60℃. The thermal cycling conditions were 94℃ for 3 minutes followed by 30 cycles of 94℃ for 30 seconds, 55℃–60℃ for 30 seconds, and 72℃ for 30 seconds, with a final extension at 72℃ for 10 minutes. The final PCR products were run on 1.2% (w/v) agarose gel and visualized under a blue-light trans-illuminator.
Results
MSCs were outgrown from the periphery of tissue explants within 5–7 days of culture. The cells took 2–3 more weeks to grow to confluence in 25-mm2 tissue culture plates. Two types of cells, spindle and polygonal in shape, accounted for most of the initial observations, and all showed fibroblast-like morphology at confluence. The cells were positive for the MSC-specific markers CD44, CD73, CD90, and CD105.
Germ cell specification was assessed by two approaches: the expression of molecular markers and changes in morphology. Induction of cells with ATRA drastically reduced the rate of proliferation, and slight changes in morphology (short and round) were observed with time (Figure 1). Gene expression studies by reverse-transcription PCR indicated the presence of at least a minor subpopulation of cells with SSC characteristics among the Wharton's jelly-derived mesenchymal stem cells (WJ-MSCs). This was confirmed by the behaviour of Plzf and Oct4, which are accepted markers for pluripotent stem cells and progenitor spermatogonia. Expression of these factors was prominent in WJ-MSCs, but became less intense after induction with ATRA, confirming the differentiation of the cells. Stra8 is predominantly expressed in the premeiotic stage, and meiotic entry of our cell population was confirmed by the presence of specific bands during the induction period. The expression pattern of Scp3, which is involved in the recombination and segregation of meiotic chromosomes, was also changed or highly expressed in cells after treatment. Similarly, the postmeiotic spermatid markers Acr (which helps with the localization of acrosin) and Prm1 (which indicates chromosome condensation) were expressed only after ATRA induction, as shown in Figure 2. However, germ cell morphology was not distinct at this stage.
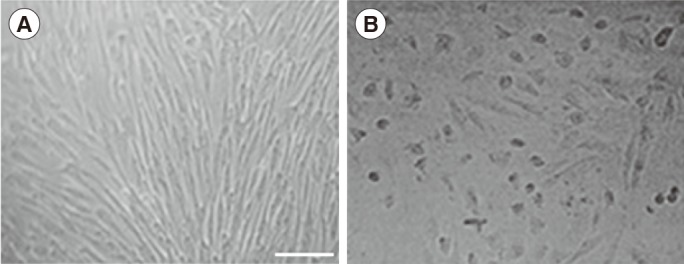
Morphological changes of mesenchymal stem cells after all-trans retinoic acid treatment. (A) The cells were fibroblastic in appearance before treatment. (B) Short and round cells were more common in the treated sample. Scale bar, 50 µm.

Expression pattern of stem and germ cell markers before (a) and after (b) treatment with all-trans retinoic acid for 14 days. The expression of stem cell markers (Oct4, Plzf) decreased, while the expression of premeiotic (Stra8), meiotic (Scp3) and postmeiotic (Acr, Prm1) marker genes increased.
Further culture of ATRA-induced putative germ cells with Sertoli cell-conditioned medium led to the emergence of a minor subpopulation of morphologically detectable germ cells. A few round colonies of cells sharing the features of embryoid body-like structures emerged in each well as the culture period was prolonged. A number of round cells with different sizes were scattered around the periphery of these colonies, and most of them were loosely attached or released into the medium (Figure 3).

Emergence of embryoid body-like structures after culturing of all-trans retinoic acid-induced putative germ cells with Sertoli cell-conditioned medium. Most of the cells scattered around the periphery had a morphology similar to that of germ cells (scale bar, 100 µm).
Microscopic observation of a trypsinized cell suspension under a high-power field revealed the presence of four stages of germ cells, including round and elongating spermatids. Of the four stages of acrosome development, two stages (the Golgi phase and the cap phase) were clearly visible in secondary spermatocytes. The clarity of the margins was satisfactorily observed with periodic acid-Schiff stain. Acrosome granule and acrosome vesicle-like structures were identified in around 5% of cells. However, only 0.5%–1% of cells in our population were round spermatids, and elongating cells were rare (Figure 4).
Discussion
Considerable success has recently been achieved in the field of in vitro spermatogenesis, especially with animal models. Good results have been reported from combined in vitro-in vivo models using SSCs as the starting cell population. Simulating this process with humans has ethical and clinical limitations. Furthermore, using SSCs has limited value in achieving the immediate goal of infertility treatment, as some men lack SSCs in their seminiferous tubules. As such, numerous attempts have been made to differentiate male germ cells from somatic stem cells in vitro for decades. This is conceptually possible because the specification of germ cells in mammals occurs independently of germ plasma inheritance, and PGCs are specified in the proximal epiblast region in response to signals emanating from extra embryonic endoderm [2].
Toyooka et al. [25] observed sperm formation from Bmp4-induced mouse ESCs transplanted into recipient testes. Easley et al. [17] obtained a roughly 5% proportion of haploid cells by direct differentiation of human ESCs and iPSCs in monolayer cultures. In a recent study, sperm were observed in recipient testes after the direct transplantation of adipose tissue MSCs into rat testes [28]. Differentiation of germ cells from ESCs seems to be more efficient than from adult stem cells in complete in vitro models [12]. Transcriptional and phenotypic similarities between ESCs and germ-line stem cells (GSCs) and the need for additional factors for adult stem cells to differentiate into mature germ cells are possible explanations for this observation [2930]. The majority of studies have ended up at the level of PGCs or round spermatids, and none of the cell types (embryonic or adult stem cells) have appeared to support complete transmeiotic differentiation. The sperm and live pups produced through an in vitro-in vivo model by Nayernia et al. [5] were only partially successful due to abnormal methylation patterns.
In the present study, we employed a simple two-step method to differentiate male germ cells from WJ-MSCs using retinoic acid and Sertoli cell-conditioned medium. Retinoic acid is a small polar molecule that can easily diffuse and bind to nuclear retinoic acid receptors, thereby controlling the expression of the retinoic acid-responsive gene Stra8. Therefore, retinoic acid has been used in many studies to efficiently differentiate male germ cells from stem cells. Our results are consistent with those of previous studies [1417], and indicate that MSCs can transdifferentiate into germ cells and undergo meiosis under appropriate culture conditions. Meiosis is one of the critical steps in spermatogenesis, and retinoic acid is also a well-known meiotic inducer [18]. An early study has confirmed the optimal concentration of retinoic acid and duration of induction to initiate meiosis to be 10 µM and 5 days, respectively, in embryoid body culture systems [6]. However, variations are expected depending on the culture system used, the type of cell, and the duration of culture. The absence of haploid cells was noted by Jia et al. [31] after inducing myoblast cells with 10 µM ATRA for 8 days.
The sequential pattern of gene expression by germ cells undergoing differentiation is the key factor for assessing whether the process of spermatogenesis has been accurately recapitulated. The expression of meiotic and post-meiotic genes with 10 µM ATRA confirmed the meiotic progression of cells in our study. Plzf and Oct4 are essential for stem cell self-renewal and are known markers of undifferentiated spermatogonia [32]. The proper disappearance of the expression of these factors with ATRA induction was observed in this study. Stra8 is predominantly expressed in premeiotic germ cells, and low or absent levels of its mRNA are reported in postmeiotic cells. Retinoic acid can directly stimulate the Stra8 gene in premeiotic germ cells without support from somatic cells [33]. Slight Stra8 expression was observed in our study after 2 weeks of induction, indicating the presence of a mixed population of meiotic and postmeiotic cells. Acr and Prm are expressed at the time of acrosome formation and the replacement of histones with protamine at the time of nuclear condensation, respectively [34]. Prominent expression of these genes was confirmed in the present study, indicating the emergence of postmeiotic germ cells. However, appropriate segregation of chromosomes may be unlikely even if the correct expression pattern is exhibited, as suggested by Clark et al. [2]. Therefore, we extended the culture with Sertoli cell-conditioned medium in the presence of adult male serum for 3 more weeks. It is suggested that FBS in the culture medium may not be supportive for the final stage of male germ cell maturation. Knockout serum replacement-containing media has been suggested for promoting the maturation of male germ cells to the elongated spermatid stage, and it may help to reduce the batch-to-batch variations experienced with FBS [1635]. We assumed that adult male serum may contain favourable substances for spermatogenesis, specifically enriched amounts of FSH and testosterone.
Our ability to go beyond the round spermatid stage, to the formation of tail bud-like structures, was most likely due to the close resemblance of WJ-MSCs to GSCs or the occurrence of a subpopulation of stem cells sharing GSC characteristics. Adult male serum, which is rich in FSH, testosterone, and the essential factors provided by Sertoli cell-conditioned medium in the form of growth factors and nutrients, may have further supported this progress. Although the physical support provided by Sertoli cells may not be an absolute requirement, those biochemical factors may influence the efficiency of germ cell formation and progression through their stages of development. However, germ cells undergoing developmental progression were only observed to a very limited extent. The emergence of advanced stages of germ cells without direct Sertoli-germ cell contact is an interesting finding of this study. We hypothesize that the primary effect of direct Sertoli-germ cell contact is to regulate the speed of germ cell development and to synchronize the epithelial cycles along the seminiferous tubules, rather than to support differentiation. Germ cell specification may be linked to cell fate commitment, leading to gametogenesis [25]. The reason for the rapid differentiation of germ cells outside their niche is speculated to be the abrogation of checkpoints at different developmental stages [20]. Providing the precise arrangement of cells in vitro is difficult, and according to Reda et al. [22], germ cells tended to migrate out of the cell colonies and die within a few days in mixed rat testicular cells maintained in three-dimensional cultures, which may have been due to the lack of proper Sertoli cell support. The factors supporting progression through spermiogenesis must still be clarified, as the efficiency of this process is far less than is to be expected. Gohbara et al. [23] observed rare events of haploid cells, but not sperm, even in a modified organ culture system that included all cell types in the germ cell niche. In addition to the topological arrangement of cells in the niche environment, this process requires endocrine and auto/paracrine regulation, as well as direct cell-cell interactions [11]. Sertoli cells help to maintain the viability of spermatogonia, induce meiosis through intercellular junctions, and assist in the endocrine and paracrine regulation of cells [21]. FSH is a prerequisite for completing meiosis and spermiogenesis, and testosterone potentiates the effects of FSH by preventing Sertoli cells from undergoing apoptosis in in vitro culture systems [20]. However, direct contact of spermatids with Sertoli cells is minimal at this stage, and the process may continue through the joint action of Sertoli-germ cells to regulate the immediate environment [36]. Nonetheless, the contributions of Leydig cells and myoid cells are poorly defined.
We propose that the failure to achieve spermiogenesis in our study was due to the lack of regulatory factors provided by different supportive cells. GSCs may have an inherent ability to respond to signals from the gonadal microenvironment [30]. The precise duration of time and temperature needed to complete spermiogenesis in vitro are also not well defined although most studies have used 2–4 weeks at 37℃. Abu Elhija et al. [26] obtained normal spermatozoa from mouse SSCs after 4 weeks in a soft agar culture system with somatic compartments. It has been reported that 34℃ was favourable for mouse spermatogenesis [23]. These issues must be addressed in relation to human somatic stem cell-derived germ cells in the future. Human WJ-MSCs were successfully transdifferentiated into postmeiotic germ cells using a simple two-step induction protocol. Although haploid cells were achieved, up to the initial stage of tail formation, the efficiency of transformation through spermiogenesis was very low. The regulatory factors needed at this stage must be clarified further.
Notes
This work was supported by the Higher Education for the Twenty-First Century (HETC) project under the grant KLN/O-Med-R/1.
Conflict of interest: No potential conflict of interest relevant to this article was reported.
