Introduction
Anti-Müllerian hormone (AMH) is a protein produced by granulosa cells surrounding follicles and is encoded by the
AMH gene [
1]. AMH is expressed during the reproductive years and it recruits follicles from the antral follicle pool [
2,
3]. In healthy women, AMH is detectable from 3 months of age and increases linearly until 8 years of age. AMH levels remain constant through adolescence and do not vary during the menstrual cycle [
4,
5,
6]. Generally, high serum AMH concentrations correlate with high antral follicle counts and a large number of resting primordial follicles in reproductive-age women [
7]. The value of AMH levels in
in vitro fertilization (IVF) treatment is to predict the ovarian response to gonadotropin [
8]. However, the serum AMH concentration does not reflect the quality of retrieved oocytes nor predict the success rate of already established pregnancies after IVF [
9,
10,
11].
Serum AMH levels are frequently measured as part of the initial work-up to assess ovarian reserve. Clinicians are often faced with patients with low serum AMH levels compared to their counterparts within the same age group. Physicians and patients with low serum AMH levels are anxious about loss of time to achieve pregnancy. Therefore, they tend to consider assisted reproductive technology (ART) treatment earlier than would usually be the case, even if the patient is young, has no other reason for infertility, or has a short duration of attempting pregnancy. Very limited data exist on the correlation between AMH and natural conception because measuring the natural pregnancy rate is very difficult [
12]. Steiner et al. [
13] reported that the natural pregnancy rate was diminished in women with low AMH levels (≤0.7 ng/mL) at a late reproductive age compared to women with normal AMH levels. However, Hagen et al. [
12] showed that low AMH did not predict a reduced natural pregnancy rate in patients in their mid-20s. No study has investigated the effects of low AMH on the natural pregnancy rate of women in their early 30s. In previous studies, time to pregnancy (TTP) and the number of menstrual cycles required before achieving pregnancy have been used to evaluate the correlation between AMH and the natural pregnancy rate [
13,
14].
We hypothesized that patients younger than 35 years of age with unexplained infertility with low ovarian reserve would have a comparable pregnancy rate to their counterparts with normal ovarian reserve, even without undergoing ART. We evaluated the pregnancy rate and TTP after timed coitus (TC) with or without superovulation in infertile young women who were younger than 35 years of age and had low serum AMH levels. Furthermore, we investigated the predictors of achieving pregnancy in these infertile women.
Methods
This study was approved by the Institutional Research Ethics Committee of Cheil General Hospital and Women's Healthcare Center (No. CGH-IRB-2015-42). Informed consent could not be obtained, as this study involved a retrospective medical record analysis.
We reviewed the medical records of 324 patients with infertility to identify serum AMH levels, hysterosalpingography (HSG) results, and semen analysis results from February 1, 2010 to December 31, 2012. We evaluated 202 patients who were younger than 35 years (range, 27–34 years), had unilateral or bilateral patent tubes on HSG, and whose partners had a normal semen analysis by the 2010 World Health Organization criteria [
15]. The patients had regular menstruation (at intervals of 24–45 days). Ninety-eight infertile women with normal serum AMH levels were used as controls, and 75 women with low serum AMH levels (5th≤&<25th percentile) and 29 women with very low serum AMH levels (<5th percentile) were evaluated as the study population. The normal serum AMH concentration was 2.5–6.65 ng/mL for patients ≤31 years of age and 2.0–5.7 ng/mL for patients 32–34 years of age. Low serum AMH levels were defined as <2.5 ng/mL for patients ≤31 years of age and <2.0 ng/mL for patients 32–34 years of age. Very low AMH levels were defined as <1.19 ng/mL for patients ≤31 years of age and <0.60 ng/mL for patients 32–34 years of age [
16]. We used the following exclusion criteria: (1) known causes of infertility such as uterine factors (intrauterine synechia, septate uterus, and double uterus), (2) bilateral tubal obstruction, (3) peritoneal factors such as endometriosis or peritubal adhesion, (4) polycystic ovary syndrome, (5) an irregular menstruation cycle (menstrual duration: <24 days or >45 days), and (6) a partner with an abnormal semen analysis.
At the first clinic visit, transvaginal sonography (TVS) was performed to exclude uterine and pelvic infertility factors. Patients provided blood samples for tests of basal follicle-stimulating hormone (FSH), luteinizing hormone (LH), estradiol, and AMH levels on the second or third day of the menstrual cycle. All samples were collected from an antecubital vein and analyzed in the same laboratory. The serum AMH levels were measured with an enzyme immunoassay using an AMH/Müllerian inhibiting substance enzyme immunoassay kit, which is a two-step immunological sandwich-type assay (Immunotech; Beckman Coulter, Marseille, France). The measurement range of the assay is from 0.1 to 7.8 ng/mL; the intra- and inter-assay coefficients of variation were 12.3% and 14.2%, respectively. To evaluate tubal patency, HSG was performed between days 9 and 12 of the menstrual cycle. The semen analysis of the partner was performed at the same time.
To monitor ovulation in a natural cycle (n=80), follicle growth was evaluated using TVS on days 11–14 of the menstrual cycle. When the follicular diameter was greater than 18 mm with profound cervical mucus or a urinary LH test was positive on the day of the office visit, TC was recommended two times on that day and the next day. For augmented ovulation cycles (n=67), we used clomiphene citrate (CC; Clomid 50–100 mg/day for 5 days; Clomifene, Young Poong Pharmaceutical, Incheon, Korea) at first, and an aromatase inhibitor (2.5 mg/day for 5 days; Femara, Novartis, Geneva, Switzerland) was also used in patients with a thin endometrium who had previously received CC treatment. For superovulation cycles (n=55), we used CC or Femara combined with a low dose (75–150 IU) of recombinant FSH (follitropin; Gonadopin, Dong-A Pharmaceutical, Seoul, Korea; Follitrope, LG Life Science, Seoul, Korea) or human menopausal gonadotropin (menotrophin; Menopur, Ferring, Geneva, Switzerland). When the dominant follicular diameter was greater than 18–20 mm, 5,000 IU of human chorionic gonadotropin (IVF-C, LG Life Science) was administered intramuscularly and TC was recommended two times on the next (second) day and third day after the human chorionic gonadotropin injection. Ovulation was confirmed through ultrasonography within 2 or 3 days and by an elevated basal body temperature. Follow-up appointments were scheduled every month, and the duration of follow-up was up to 29 months.
Pregnancy was defined as the confirmation of an intrauterine gestational sac through TVS. Miscarriage was defined by fetal demise or the absence of fetal heart tones before the 20th week of pregnancy. The TTP was calculated from the month when pregnancy was attempted to the month of gestational sac confirmation.
Statistical analysis was performed with IBM SPSS ver. 21.0 (IBM Corp., Armonk, NY, USA). Continuous variables are presented as mean±standard deviation, and categorical variables are expressed as number or percentage. The normal distribution of the data was verified using the Kolmogorov-Smirnov test. If the data were normally distributed, the independent sample t-test was used to compare basal characteristics (age, type of infertility, parity, menstrual interval, body mass index [BMI], paternal age, and semen analysis results).
Categorical variables (pregnancy rate and miscarriage rate) were compared using the chi-square test or the Fisher exact test. The Pearson correlation coefficient was calculated to estimate the correlations between pregnancy and multiple variables (age, primary infertility, parity, infertility duration, BMI, and serological factors). Logistic regression analysis was performed to evaluate the predictive power of independent variables for achieving pregnancy. Kaplan-Meier curves were used to illustrate the cumulative pregnancy rate until 18 months according to the AMH group. The p-values less than 0.05 were considered to indicate statistical significance.
Results
Age, type of infertility, parity, menstrual interval, BMI, paternal age, and semen analysis results (sperm count, motility and morphology) were similar in the women with normal and low AMH levels (
Table 1). The basal serum FSH levels on the second or third day of the menstrual cycle were significantly different between the normal AMH and very low AMH groups (7.4±1.9 mIU/mL vs. 10.6±7.5 mIU/mL,
p=0.02).
The mean TTP was not statistically significantly different between women with normal AMH and low AMH levels (6.9 months vs. 8.9 months,
p=0.192). However, the TTP in the very low AMH group was significantly longer than in the normal AMH group (13.1 months vs. 6.9 months,
p=0.03) (
Table 1).
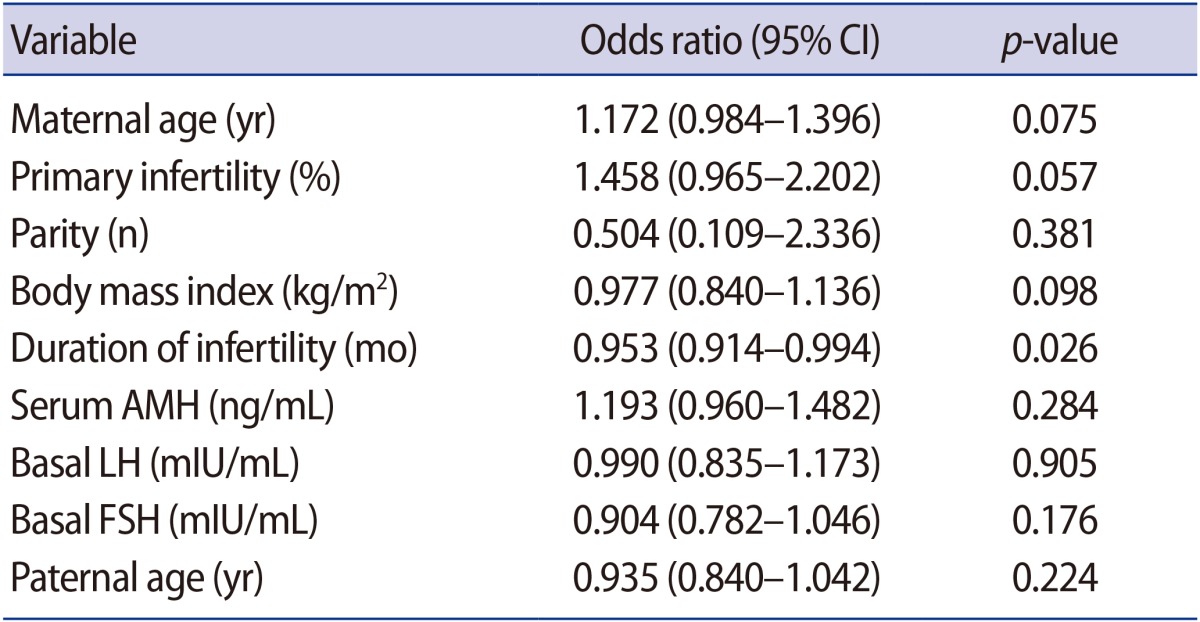
The pregnancy rate after TC in the two groups was similar (normal AMH group vs. low AMH group: 43.9% [43/98] vs. 41.3% [31/75], respectively,
p=0.759). The miscarriage rate was higher in the low AMH group, but this trend did not reach statistical significance (normal AMH group vs. low AMH group: 14.0% [6/43] vs. 29.0% [9/31], respectively,
p=0.146). The pregnancy rate in the very low AMH group was lower than in the normal AMH group (27.6% [8/29] vs. 43.9% [43/98], respectively,
p=0.135) and the miscarriage rate in the very low AMH group was higher than in the normal AMH group (37.5% [3/8] vs. 13.9% [6/43], respectively,
p=0.137), without statistical significance (
Figure 1).
When comparing the pregnancy rate after TC according to the ovulation method, no statistically significant differences were found (
Table 2). When correlations were evaluated between achieving pregnancy and associated factors (maternal age, type of infertility, parity, BMI, infertility duration, serum AMH level, basal LH and FSH level, and paternal age) through logistic regression analysis, the duration of infertility was found to be negatively correlated with achieving pregnancy (odds ratio, 0.953; 95% CI, 0.914–0.994;
p=0.026) (
Table 3).
The cumulative live birth rates (CLBR) were 26.5%, 17.3%, and 4.0% at 6 months and 37.7%, 29.3%, and 4.0% at 1 year in the normal AMH group, low AMH group, and very low AMH group, respectively. The CLBR within 18 months was not significantly different between the normal AMH group and the low AMH group for patients younger than 35 years (41.8% vs. 37.3%,
p=0.915). However, the 18-month CLBR in the very low AMH group was lower than in the normal AMH group (17.2% vs. 41.8%,
p=0.090) (
Figure 2).
Discussion
There is a considerable amount of published data on the correlation between serum AMH and pregnancy outcomes in women treated with ART [
8,
17]. To the best of our knowledge, very few data have been published on the natural pregnancy rate according to serum AMH levels [
13,
18]. Hagen et al. [
12] reported that low serum AMH levels in women who had no history of parity did not predict a reduced natural pregnancy rate for patients in their mid-20s. In the results of the present study, the pregnancy rate after TC with or without superovulation was not different between the low AMH and normal AMH groups. This is similar to the results found by Hagen et al. [
12].
However, the CLBR in the very low AMH group was somewhat lower than in the normal AMH group although without statistical significance. Moreover, the TTP in the very low AMH group was significantly longer than in the normal AMH group. Steiner et al. [
13] reported that the natural pregnancy rate in patients with low serum AMH levels (≤0.7 ng/mL) was significantly lower than in patients with higher AMH levels in healthy women aged 30–42 years. They also suggested that women with a higher serum FSH level (≥10 mIU/mL) at the early follicular phase had a lower natural pregnancy rate [
13]. The results of the present study showed that patients in the very low AMH group had higher serum FSH levels (7.4±1.9 mIU/mL vs. 10.6±7.5 mIU/mL,
p=0.002). These results suggest that serum AMH levels and basal serum FSH levels must be considered jointly to predict the possibility of achieving pregnancy [
10]. We also suggest that for relatively young patients who have very low ovarian reserve (serum AMH <1.19 ng/mL in patients ≤31 years of age and <0.60 ng/mL in those 32–34 years of age) without any infertility factors, active management should be recommended to avoid loss of time to get pregnant.
The miscarriage rate has been reported to be approximately 8%–21% in women younger than 35 years [
19]. In the present study, the miscarriage rate was higher in the very low AMH group and the low AMH group. Several studies have reported a higher incidence of aneuploid blastocysts in women with low ovarian reserve than in women with normal ovarian reserve when they were treated with IVF-embryo transfer [
20]. Our results can be interpreted in the light of the extensive data published on this issue.
The mean TTP and CLBR until 18 months were not significantly different between the low AMH group and the normal AMH group. Based on the results of our study, conservative management, such as TC with or without superovulation, should be considered in infertile women with low serum AMH levels (1.19≤&<2.5 ng/mL in patients ≤31 years of age and 0.06≤&<2.0 ng/mL in those aged 32–34 years) who are younger than 35 years of age and have no other causes of infertility. These results are supported by those of the studies by Tremellen and Kolo [
21] and Streuli et al. [
18]. Tremellen and Kolo [
21] reported that serum AMH levels did not affect the likelihood of a live birth in women who were treated with intrauterine insemination. Streuli et al. [
18] concluded that serum AMH levels were not correlated with the effective TTP (between 3 and 6 months).
In the present study, the duration of infertility showed a significant negative correlation with achieving pregnancy in women younger than 35 years. Moreover, patients with a long duration of infertility tend to pursue more active management, and they have no room to wait for pregnancy naturally. Thus, active infertility treatment such as ART, including intrauterine insemination, should be considered for women with a long history of infertility.
Universal age-specific reference values of serum AMH do not yet exist [
22]. We used the normal AMH level (25th–75th percentiles of age-specific values) from our previously published data [
16]. The range of normal AMH is somewhat wide, making it difficult to define normal serum AMH levels. However, these results regarding age-specific AMH levels are similar to those reported by La Marca et al. [
23].
In conclusion, conservative management, such as TC with or without superovulation, should be considered in infertile women with low serum AMH levels who are younger than 35 years and have no other causes of infertility. However, for women with a long duration of infertility or with very low serum AMH levels, active treatment to achieve pregnancy should be considered.
Figure 1
Pregnancy rate (A) and miscarriage rate (B) after timed coitus in the normal, low, and very low anti-Müllerian hormone (AMH) groups.
Figure 2
Cumulative live birth rates of infertile women under age 35 years in the normal anti-Müllerian hormone (AMH), low AMH, and very low AMH groups. (A) Normal AMH group vs. low AMH group (p=0.140), (B) normal AMH group vs. very low AMH group (p=0.051).

Table 1
Clinical characteristics and time to pregnancy in the normal AMH, low AMH, and very low AMH groups

Table 2
Clinical characteristics and CPR according to the ovulation method
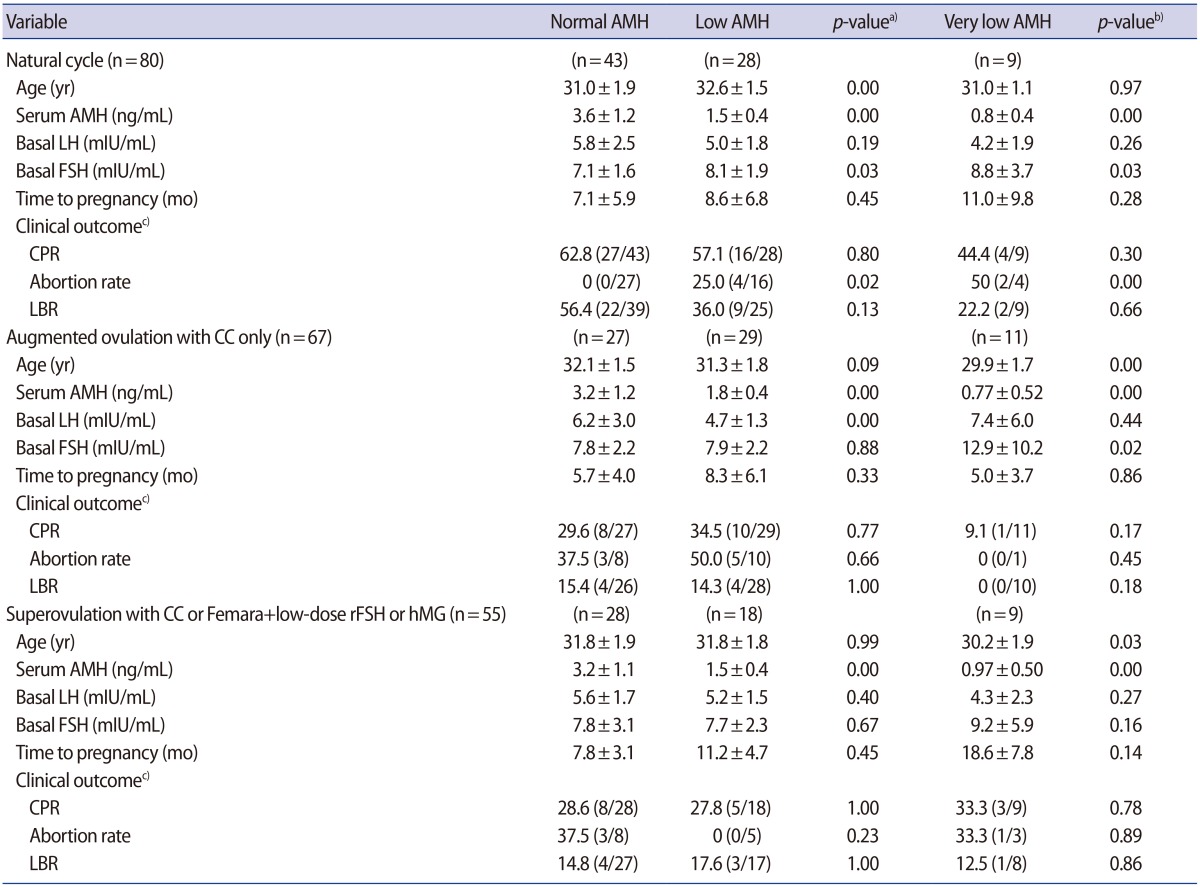
Table 3
Logistic regression analysis of the factors associated with natural pregnancy