GnRH antagonist multiple dose protocol with oral contraceptive pill pretreatment in poor responders undergoing IVF/ICSI
Article information
Abstract
Objective
To investigate the effectiveness of GnRH antagonist multiple-dose protocol (MDP) with oral contraceptive pill (OCP) pretreatment in poor responders undergoing IVF/ICSI, compared with GnRH antagonist MDP without OCP pretreatment and GnRH agonist low-dose long protocol (LP).
Methods
A total of 120 poor responders were randomized into three groups according to controlled ovarian stimulation (COS) options; GnRH antagonist MDP after OCP pretreatment (group 1), GnRH antagonist MDP without OCP pretreatment (group 2) or GnRH agonist luteal low-dose LP without OCP pretreatment (group 3). Patients allocated in group 1 were pretreated with OCP for 21days in the cycle preceding COS, and ovarian stimulation using recombinant human FSH (rhFSH) was started 5 days after discontinuation of OCP.
Results
There were no differences in patients' characteristics among three groups. Total dose and days of rhFSH used for COS were significantly higher in group 3 than in group 1 or 2. The numbers of mature oocytes, fertilized oocytes and grade I, II embryos were significantly lower in group 2 than in group 1 or 3. There were no significant differences in the clinical pregnancy rate and implantation rate among three groups.
Conclusion
GnRH antagonist MDP with OCP pretreatment is at least as effective as GnRH agonist low-dose LP in poor responders and can benefit the poor responders by reducing the amount and duration of FSH required for follicular maturation.
Introduction
The management for poor responders with diminished ovarian reserve is still a challenge, although many studies have been performed to seek for a method of efficient ovarian stimulation for infertile women with reduced ovarian reserve. There have been many studies assessing various controlled ovarian stimulation (COS) regimens to improve the outcome of poor responders undergoing IVF, no particular protocol was demonstrated to be best suited for all such patients [1]. GnRH agonist long protocol (LP) has been a standard COS method since it was introduced in assisted reproduction in the late 1980's to prevent premature LH surge and luteinization [2], and GnRH agonist low-dose regimens was advocated as one of important protocols for poor responders in terms of improvement in COS response and reduced duration and dose of gonadotropin stimulation [3,4]. More recently, GnRH antagonists have been administered to poor responders during COS with inconsistent results [5-9]. Although a limited number of randomized controlled trials compare the efficacy of GnRH antagonist protocol and the agonist long protocol for COS in poor responders undergoing IVF, a meta-analysis by Griesinger et al. [10] demonstrated that GnRH antagonist protocol results in significantly more cumulus oocyte complexes (COCs) and similar clinical outcome. COS using GnRH antagonists is also likely to become one of the treatment options for poor responders.
After the introduction of GnRH antagonists in COS, oral contraceptive pill (OCP) has been used for cycle scheduling purpose. OCP is effective in allowing prediction of timing events in an IVF cycle regarding scheduling and is frequently used before IVF and may influence IVF outcome [11,12]. In a randomized controlled trial by Kolibianakis et al. [13] and a meta-analysis by Griesinger et al. [14], a significant difference in ongoing pregnancy rates between patients who received OCP pretreatment and those who did not was not found. However, subjects for these studies were not poor responders and the available randomized studies on OCP pretreatment in poor responders undergoing IVF have not been reported. It is thought that OCP pretreatment in GnRH antagonist cycles can result in different ovarian response to COS and IVF results in poor responders, compared with normal responders.
The present study was performed to investigate the effectiveness of GnRH antagonist multiple-dose protocol (MDP) with OCP pretreatment in poor responders undergoing IVF/ICSI, compared with GnRH antagonist MDP without OCP pretreatment and GnRH agonist low-dose LP.
Methods
1. Patient population
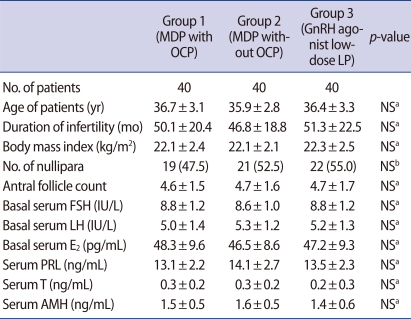
A total of 120 poor responders, aged 28-41 years, who were defined as patients with repeated day 3 levels of FSH>8.5 mIU/mL [15], and/or antral follicle count (AFC)≤5 [16,17], were recruited from a university-based infertility clinic at the Asan Medical Center, Seoul, Korea. The Institutional Review Board of our center approved the study and all patients provided written informed consent. Patients were randomized on the basis of a computer-generated list to three groups according to COS options; GnRH antagonist MDP after OCP pretreatment (group 1), GnRH antagonist MDP without OCP pretreatment (group 2) or GnRH agonist luteal low-dose long protocol without OCP pretreatment (group 3). The sequence of allocation to the three groups was provided to the investigating physicians and randomization was performed as planned according to the randomization list order. All three groups consisted of 40 cycles initiated corresponding to 40 patients, respectively. A probability by power calculation for sample size determination was 81%.
All patients were in good health with normal hepatic, and renal functions with a body mass index of between 19 and 30 kg/m2. None of subjects had ever taken any infertility medications (clomiphene and/or gonadotropins) within the preceding three months. Patients with polycystic ovary syndrome according to Rotterdam definition were excluded from this study. Hormonal profiles (serum levels of basal FSH, LH, E2, prolactin, testosterone and antimϋllerian hormone) and the number of basal antral follicle in ultrasonogram were measured on the 2nd or 3rd day of menstruation in all patients.
2. Ovarian stimulation protocols
All patients allocated in group 1 were pretreated identically with Minivlar (ethinyl estradiol 0.03 mg and levonorgestrel 0.15 mg; Schering AG, Berlin, Germany) for 21 days in the cycle preceding COS. Five days after OCP discontinuation for group 1 and on cycle day 3 for group 2, ovarian stimulation was commenced using recombinant human FSH (rhFSH, Gonal-F; Merck Serono SA, Geneva, Switzerland) of 225 IU/day after establishing ovarian and uterine quiescence using vaginal ultrasound. The rhFSH dose was adjusted according to ovarian response, every 3-4 days. GnRH antagonist (Cetrotide 0.25 mg; Merck Serono SA) was started when the leading follicle reached 14 mm in average diameter, and was continued daily until the day of hCG administration. A recombinant hCG (rhCG, Ovidrel; Merck Serono SA) of 250 µg was injected to induce follicular maturation when one or more follicles reached a mean diameter of ≥18 mm. Oocyte retrieval was performed 35 to 36 hours after hCG injection and one to four embryos were transferred into the uterus on the third day after oocyte retrieval. Vaginal progesterone gel (Crinone 8%, 90 mg, Merck Serono SA) once daily was administrated from the day of oocyte retrieval, for luteal support. In group 3, the daily injection of GnRH agonist (Decapeptyl 0.1 mg; Ferring, Malmö, Sweden) was initiated from the midluteal phase and continued until menses followed by a dose reduction to 0.05 mg daily. GnRH agonist 0.05 mg daily was continued up to day of hCG administration. Ovarian stimulation using rhFSH, oocyte retrieval and luteal support were performed in the same manner with GnRH antagonist MDP.
The serum level of β-hCG was measured 11 days after embryo transfer (ET). Measurement of β-hCG was performed by radioimmunoassay using a hCG MAIAclone kit (Serono Diagnostics, Woking, UK); interassay and intraassay variances were less than 10% and 5%, respectively.
3. Outcome measures
Primary efficacy endpoint was the number of mature oocytes retrieved. Secondary efficacy variables included total amount and days of rhFSH administered, the numbers of fertilized oocytes and grade I, II embryos, implantation rate, clinical pregnancy rate per cycle, and live birth rate per cycle.
Clinical pregnancy was defined as the presence of a gestational sac by ultrasonography 5 weeks after oocyte retrieval, while miscarriage rate per clinical pregnancy was defined as the proportion of patients who failed to continue development before 20 weeks of gestation in all clinical pregnancies. Live birth was defined as the delivery of a fetus with signs of life after twenty four completed weeks of gestational age.
4. Statistical analysis
The mean value was expressed as the mean±SD. Analysis of variance was used to compare the mean values among three groups. Chi-square test and Fisher's exact test were used for the comparisons of fraction. Statistical significance was defined as p<0.05. All analyses were performed using the SPSS statistical package for Windows, ver. 12.0 (SPSS Inc, Chicago, IL, USA).
Results
There were no differences in the age of patients, duration of infertility, body mass index, the proportion of nullipara, AFC and basal endocrine profile among three groups (Table 1).
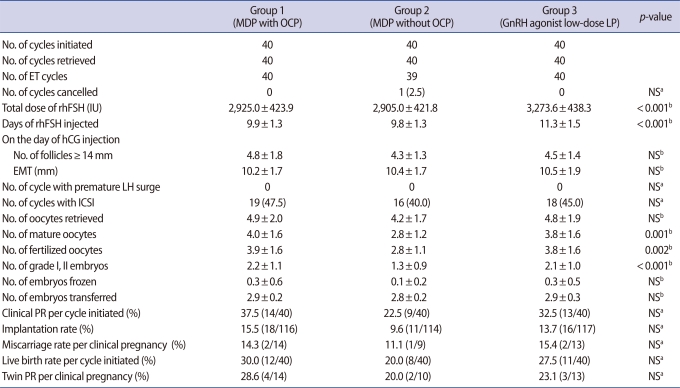
In group 1 and 3, no patient was lost to follow-up or withdrawn from the study and there were no cancellation of cycle by premature LH surge or any other reasons. In group 2, 1 out of 40 cycles initiated (2.5%) was cancelled before ET, because no oocytes were obtained despite a follicular aspiration for oocyte retrieval. However, there was no significant difference in cycle cancellation rate among three groups (Table 2).
Total doses (2,925.0±423.9 IU vs. 2,905.0±421.8 IU vs. 3,273.6±438.3 IU) and days (9.9±1.3 days vs. 9.8±1.3 days vs. 11.3±1.5 days) of rhFSH required for COS were significantly higher in group 3 than in group 1 or 2 (p<0.001, p<0.001) (Table 2). The number of follicles ≥14 mm and endometrial thickness on hCG day, number of oocytes retrieved were similar among three groups, but the number of mature oocytes (4.0±1.6 vs. 2.8±1.2 vs. 3.8±1.6), fertilized oocytes (3.9±1.6 vs. 2.8±1.1 vs. 3.8±1.6) and grade I, II embryos (2.2±1.1 vs. 1.3±0.9 vs. 2.1±1.0) were significantly lower in group 2 than in group 1 or 3 (p=0.001, p=0.002, p<0.001, respectively) (Table 2). However, significant differences were not found among three groups regarding clinical pregnancy rate per cycle initiated, implantation rate and live birth rate per cycle initiated (Table 2).
Discussion
The present study evaluated the effectiveness of GnRH antagonist MDP with or without OCP pretreatment, compared with GnRH agonist low-dose LP that have been popularly used since the 1990's in poor responders undergoing IVF/ICSI. Total dose and duration of rhFSH used for COS were significantly higher in GnRH agonist low-dose LP group than in GnRH antagonist MDP groups, which suggests the advantage of GnRH antagonist protocol. It is well known advantage of GnRH antagonist protocols compared with GnRH agonist LP, and Howles et al. [18] reported that the GnRH antagonists offer comparable therapeutic efficacy to agonists and have a number of potential advantages over agonists for use in COS protocols, such as avoiding the initial "flare-up" of LH, shortening the overall treatment period and reducing the risk of OHSS and menopausal side effects in women of advanced maternal age. In group 1, the numbers of mature oocytes, fertilized oocytes and grade I, II embryos were comparable to those in group 3, whereas these were significantly lower in group 2. This implicates OCP pretreatment in GnRH antagonist MDP is effective in improving the ovarian response to COS and IVF outcome with similar level to GnRH agonist low-dose LP. In patients with elevated basal serum FSH levels by diminished ovarian reserve, antral follicle sizes during the early follicular phase are often markedly heterogeneous because FSH-sensitive follicles are early exposed to gradient FSH concentrations during the preceding luteal phase. Therefore, in poor responders, ovarian stimulation without pituitary suppression is likely to induce asynchronous follicular development and a limited number of dominant follicles. The COS results of present study may result from pituitary suppression prior to ovarian stimulation. Actually, a study by Fanchin et al. [19] showed that luteal FSH suppression by either estradiol or GnRH antagonists administration improves the homogeneity of early antral follicles during the early follicular phase, thereby improving ovarian response to short GnRH agonist and antagonist protocols.
In terms of clinical pregnancy rate in our study, GnRH antagonist MDP with OCP pretreatment seemed to work better than GnRH antagonist MDP without OCP pretreatment, but the difference was not statistically significant. GnRH antagonist MDP with OCP pretreatment yielded comparable IVF results and pregnancy outcome to GnRH agonist low-dose LP with fewer dose and days of rhFSH required.
Assessment of various COS regimens for poor responders is difficult because there is no universal definition for poor responder and lack of a large number of prospective randomized trials demonstrating the advantage of a specific single protocol [20]. However, low-dose GnRH agonist protocols, microdose GnRH agonist flare regimens and GnRH antagonist protocols are considered as beneficial regimens in poor responders [1,20]. Olivennes et al. [4] prospectively analyzed 98 patients with low-dose GnRH agonist protocol, which leuprolide acetate of 0.5 mg daily was initiated in the mid-luteal phase and then decreased to 0.25 mg daily after menses when gonadotropin stimulation was commenced. They reported improvement of COS results with reduced duration and doses of gonadotropin stimulation. Several studies have recently evaluated GnRH antagonist protocols comparing to alternative regimens designed for poor responders. Copperman suggested GnRH antagonist protocol in poor responders could reduce the cancellation rate of the cycle and increase the ongoing pregnancy rate, when compared with GnRH agonist microdose flare protocol [21]. Cheung et al. [22] also reported a fixed, multiple-dose GnRH antagonist protocol could be utilized for poor responders although they failed to demonstrate an overall improvement in ovarian responsiveness. These recent studies implicate the effectiveness of GnRH antagonist use in COS for poor responders.
The OCP pretreatment in IVF cycles is the simplest method for cycle scheduling, and it can be beneficial in improving ovarian responses by inhibition of intrinsic gonadotropins before ovarian stimulation. A recent meta-analysis demonstrated no significant differences between patients with and those without OCP pretreatment regarding the number of cumulus-oocyte complexes, fertilization rates and ongoing pregnancy rates [14]. However, we showed OCP pretreatment in GnRH antagonist cycles can offer advantages in IVF results in poor responders. Copperman also reported a higher proportion of patients obtained more than eight oocytes, a significantly higher pregnancy rate when OCP pretreatment was performed in poor responders [21]. It is thought that OCP pretreatment in GnRH antagonist cycles can result in different ovarian response to COS and IVF results in poor responders, compared with normal responders, and OCP pretreatment might be more advantageous in poor responders than in normal responders.
The present study demonstrated that GnRH antagonist MDP with OCP pretreatment is at least as effective as GnRH agonist low-dose LP in poor responders and can be advantageous to poor responders because of the shortened time required for follicular maturation and the diminished amount of rhFSH required to provide adequate ovarian stimulation. Therefore, GnRH antagonist protocol with OCP pretreatment might be a useful choice of COS for poor responders. Larger studies with standardized methods will be needed for confirmation of our conclusions.
Notes
No potential conflict of interest relevant to this article was reported.