Reduction of the cetrorelix dose in a multiple-dose antagonist protocol and its impact on pregnancy rate and affordability: A randomized controlled multicenter study
Article information
Abstract
Objective
To determine whether reducing the cetrorelix dose in the antagonist protocol to 0.125 mg had any deleterious effects on follicular development, the number and quality of retrieved oocytes, or the number of embryos, and to characterize its effects on the affordability of assisted reproductive technology.
Methods
This randomized controlled study was conducted at the Fertility Unit of Tanta Educational Hospital of Tanta University, the Egyptian Consultants' Fertility Center, and the Qurrat Aien Fertility Center, from January 1 to June 30, 2017. Patients' demographic data, stimulation protocol, costs, pregnancy rate, and complications were recorded. Patients were randomly allocated into two groups: group I (n=61) received 0.125 mg of cetrorelix (the study group), and group II (n=62) received 0.25 mg of cetrorelix (the control group).
Results
The demographic data were comparable regarding age, parity, duration of infertility, and body mass index. The dose of recombinant follicle-stimulating hormone units required was 2,350.43±150.76 IU in group I and 2,366.25±140.34 IU in group II, which was not a significant difference (p=0.548). The duration of stimulation, number of retrieved oocytes, and number of developed embryos were not significantly different between the groups. The clinical and ongoing pregnancy rates likewise did not significantly differ. The cost of intracytoplasmic sperm injection per cycle was significantly lower in group I than in group II (US $494.66±4.079 vs. US $649.677±43.637).
Conclusion
Reduction of the cetrorelix dose in the antagonist protocol was not associated with any significant difference either in the number of oocytes retrieved or in the pregnancy rate. Moreover, it was more economically feasible for patients in a low-resource country.
Introduction
The antagonist protocol involves controlled ovarian hyperstimulation (COH) starting from the third day of menses until the follicles reach 14 mm, at which point a gonadotropin-releasing hormone (GnRH) antagonist is given daily to prevent the luteinizing hormone (LH) surge. It is used mainly in older women, poor responders, and women with polycystic ovary syndrome [1].
The introduction of GnRH antagonists (GnRH-ant) as part of assisted reproductive technology to prevent the LH surge provided a new way of making in vitro fertilization (IVF) safer and friendlier. Unlike the indirect pituitary suppression induced by GnRH agonists (GnRH-a), GnRH-ant administration causes the immediate and dose-related inhibition of gonadotropin release by competitive occupancy of the GnRH receptors in the pituitary gland [23].
In the multiple-dose protocol, the GnRH-ant is administered continuously until the day of human chorionic gonadotropin (hCG) administration, and the minimal effective dose to prevent the occurrence of a premature LH rise was identified as 0.25 mg of cetrorelix [24].
The optimal levels of endogenous LH in GnRH-ant cycles are still a matter of debate. It may be assumed that the deep suppression of LH secretion induced by GnRH-a administration is likely to be detrimental for the follicle-oocyte complex. Low residual LH concentrations and impaired estradiol (E2) secretion with increasing antagonist doses were indeed associated with low implantation rates [5].
In contrast, a trend towards lower pregnancy rates was observed in patients with LH deficiency, documented by a low E2-to-oocyte ratio, which could be explained by the endometrial impact of low LH levels [6]. On the basis of these observations, the possibility of LH supplementation in GnRH-ant regimens was examined. Data from two randomized controlled trials showed that the addition of 75 IU of recombinant LH to recombinant FSH at GnRH-ant initiation, or starting at the initiation of stimulation, did not appear to increase pregnancy rates [7]. Similarly, no improvement in pregnancy rates was found after increasing the dose of gonadotropins by 75 IU at GnRH-ant initiation [8]. Neither study showed any evidence that low endogenous LH levels after GnRH-ant initiation were associated with a decreased probability of pregnancy in IVF cycles [8].
In a third study conducted by Baruffi et al. [9], a meta-analysis of five randomized controlled trials, significantly higher serum E2 concentrations and greater numbers of MII oocytes were observed in GnRH-ant cycles supplemented with LH, suggesting that LH may prevent any decrease in E2 levels after antagonist administration, even if there was no significant difference in the implantation and pregnancy rates.
Chang et al. [10] conducted a prospective randomized controlled trial in which a total of 58 women with a body weight of 40–50 kg were allocated into two groups. The patients in group 1 (n=28) were given a fixed cetrorelix dose of 0.2 mg/day, and the patients in group 2 (n=30) were given a fixed cetrorelix dose of 0.15 mg/day. The authors found that there were no statistically significant differences between the two groups in terms of the clinical pregnancy rate, gonadotropin dose, or incidence of ovarian hyperstimulation syndrome (OHSS).
In this study, a reduced cetrorelix dose (0.125 mg) was compared to the full dose (0.25 mg) in terms of the required dose of gonadotropins, pregnancy rate, and costs.
Methods
1. Study design and setting
This study was a non-blind, prospective, double-armed, randomized clinical trial. The study was conducted at three centers, the Educational Hospital Fertility Unit of Tanta University, the Egyptian Consultants' Fertility Center in Tanta, and the Qurrat Aien fertility Center in El- Mahalla Al-Kubra, from January 1 to June 30, 2017.
2. Eligibility
The enrolled patients (n=223) were assessed for eligibility according to the following inclusion and exclusion criteria. The inclusion criteria were: (1) age <35 years, (2) women with polycystic ovaries, (3) FSH <10 IU/mL, (4) antral follicle count >3, (5) anti-Müllerian hormone (AMH) levels ≥ 1 ng/mL, (6) body mass index ≤26 kg/m2, and (7) the presence of both ovaries. The exclusion criteria were a history of endometriosis, a history of more than two implantation failures, and a history of previous ovarian surgery.
3. Randomization and allocation
The 155 eligible women were randomly allocated into two groups. Group I (n=78) received 0.125 mg of cetrorelix (the study group) and group II (n=77) received 0.25 mg of cetrorelix (the control group). Randomization was performed by a computer program, with random numbers denoting the treatment modality. These numbers were placed in closed envelopes, and allocation was done according to these numbers. The envelope openers did not change participants' allocation.
4. Interventions
1) Stimulation protocol
All participants were treated with a GnRH-ant protocol. The patients received recombinant human FSH (150 IU subcutaneously; Gonal-F, Merck Serono, Modugno, Italy) for 5 days. Serial transvaginal sonography was performed. When a mature follicle (≥14 mm) was detected, a cetrorelix (0.125 mg/day, subcutaneously; Cetrotide, Merck Serono) was injected in group I, while 0.25 mg/day of cetrorelix was injected subcutaneously in group II. Triggering was commenced with 10,000 IU of hCG (Choriomon; IBSA, Washington, DC, USA) through an intramuscular injection when at least two follicles with a mean diameter of 18 mm were observed.
2) Ovum pickup
Transvaginal egg retrieval was done under sedation by 0.1–0.15 mg/kg/min of intravenous propofol for 3–5 minutes; it was titrated to the desired clinical effect and administered as a slow infusion or a slow intravenous injection. Ovum pickup was commenced after 36 hours for all patients.
3) Luteal support
Progesterone (vaginal suppositories, Cyclogest 400 mg; Actavis, Barnstaple, UK) was administered twice daily for luteal phase support. Luteal support started after ovum pickup and continued until evidence of cardiac pulsation was detected by ultrasonography.
4) Embryo transfer
On day 5 after oocyte retrieval, 2–3 embryos were transferred using a Labotect catheter (Labotect, Gottingen, Germany) in all patients. Serum β-hCG was assessed on day 14 after embryo transfer.
As the transfer was done on day 5; embryos were graded according to the Gardner system [11]. Good-quality embryos (grades 3–4) were defined as those in which the blastocoel completely filled the embryo, the inner cell mass was loosely grouped with several cells (grade B), and the trophectoderm had very few cells, forming a loose epithelium (grade B). Poor-quality embryos (grade 1 or 2) were defined as those with a quality lower than 3BB on day 5 [12].
5. Study outcomes and definitions
The primary outcomes included the number of oocytes retrieved, the number of developed embryos, the progesterone level on the triggering day, and the clinical/ongoing pregnancy rates. The secondary outcomes were the cost of the cycle and the occurrence of complications.
The clinical pregnancy rate was defined as evidence of pregnancy by clinical (foetal heartbeat) or ultrasound parameters (ultrasound visualization of a gestational sac, embryonic pole with heartbeat). The ongoing pregnancy rate was defined as the number of pregnancies that continued beyond 12 weeks of gestation.
6. Ethical approval and trial registration
The study was approved by the local Institutional Review Board (the ethics committee) of Tanta University (No. 31241/12/16) and was registered in the UMIN Clinical Trial Registry (ID: UMIN000027193).
7. Statistical methods
1) Sample size calculation
The sample was calculated using Epi Info ver. 7.0 (Centers for Disease Control and Prevention, Atlanta, GA, USA); H0 was postulated to indicate that the reduced cetrorelix dose would have the same effect as the full dose. Assuming a confidence level of 95%, a confidence interval of 5, and a percentage of 50%, the estimated sample was 155 patients.
2) Statistical analysis
All statistical analyses were carried out using SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA). The normality of the data distribution was checked. The mean±standard deviation was calculated for the descriptive analysis. The independent t-test was used to analyse differences. Statistical significance was considered to be indicated by p-values <0.05. According to the power analysis, the power of the study was 0.8 and α was 0.05.
Results
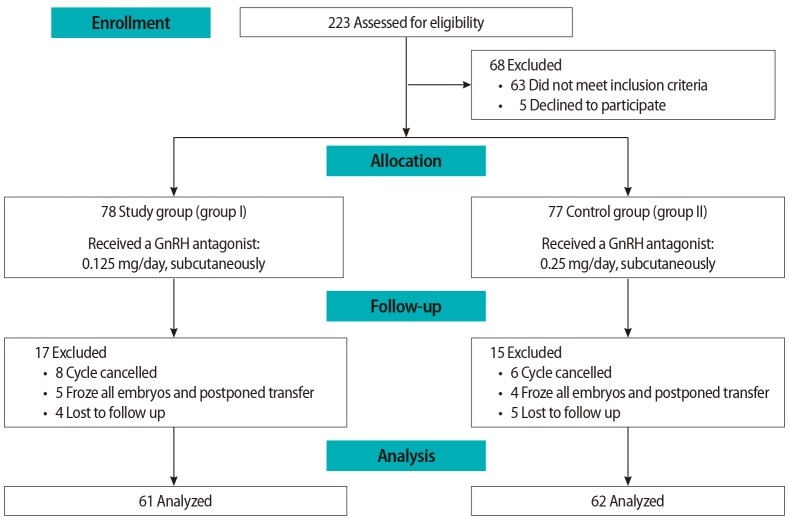
The patients enrolled at the three centers (n=223) were assessed for eligibility, and 68 patients were excluded either because they did not meet the inclusion criteria (n=63) or declined to participate (n=5). The eligible patients (n=155) were allocated into two groups: the study group (group I, n=78) and the control group (group II, n=77). Subsequently, 17 patients were excluded from group I and 15 patients from group II for other reasons, as shown in the flowchart in Figure 1.
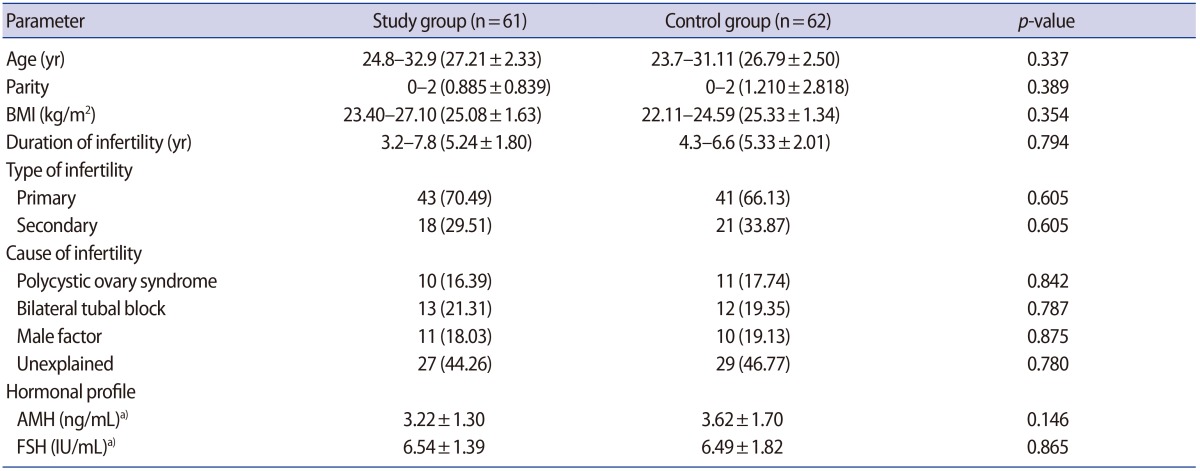
The demographic characteristics, type and duration of infertility, and hormonal profile including day 3 (AMH, FSH) were comparable in both groups. The causes of infertility in the participants are presented in Table 1.
The cycle characteristics in both groups are shown in Table 2. The mean dose of recombinant FSH was 2,350.43±150.76 IU in group I and 2,366.25±140.34 IU in group II, with no significant difference between the groups (p=0.548). The duration of stimulation (days) was 7.78±0.967 vs. 7.64±0.990 in the study and control groups, respectively (p=0.429). LH levels on the triggering day of >10 IU/mL were observed in three cases (4.9%) in the study group and in two cases (3.23%) in the control group. The number of oocytes retrieved in both groups was nearly the same: 10.132±1.340 in group I and 9.613±2.051 in group II (p=0.099). There was a nonsignificant difference in the number of developed embryos (7.213±0.777 vs. 7.174±0.638 in groups I and II, respectively), as shown in Table 2.
The endometrial thickness was 15.212±1.793 mm in group I and 14.638±1.674 mm in group II (p=0.068). The clinical pregnancy rate was similar in both groups: 29 of 61 (47.5%) in group I and 30 of 62 (48.34%) in group II (p=0.926). The ongoing pregnancy rates were also comparable in both groups (22/61 [36.07%] and 25/62 [40.32%] in groups I and II, respectively), with a p-value of 0.629. The main difference was found in the financial cost of each cycle; the cost per cycle was significantly lower in group I than in group II (US $494.66±4.079 vs. US $649.677±43.637, respectively), with a p-value of <0.001, as shown in Table 2.
There were no reported cases of OHSS in either group. Other complications are reported in Table 3. Fourteen patients in the current study were cancelled due to a poor response (n=6), a premature LH surge (n=5), or financial causes (n=3).
Discussion
Currently, GnRH-ant protocols are widely used to suppress endogenous gonadotropins to prevent a premature LH rise during COH in IVF-embryo transfer cycles. In the past, GnRH-ant was restricted to certain populations, such as older patients and those with previous poor outcomes [8].
GnRH-ant compounds were developed at the same time as GnRH-a drugs, but they were associated with a high incidence of histamine release following injection. The introduction of third- and fourth-generation GnRH-ant drugs (cetrorelix and ganirelix) minimized histamine release, enabling GnRH-ant to be used in multiple-dose regimens in women undergoing COH [13]. With GnRH-ant, rapid suppression of the pituitary gland takes place, as well as a rapid recovery of pituitary function due to the short elimination half-life, so the degree of suppression can be adjusted by changing the dose of GnRH-ant [14].
GnRH-ant protocols have clinical pregnancy and live birth rates comparable to GnRH-a long protocols, which have been the standard stimulation protocols for many years. GnRH-ant protocols have less likelihood of OHSS and a shorter gonadotropin stimulation period (less cost), making them cost-effective. Moreover, with GnRH-ant, triggering with GnRH-a could be used with minimal OHSS incidence. Al-Inany et al. [14] found that the use of GnRH-ant has moderate-quality evidence compared with long-course GnRH protocols.
Many studies have tried to determine the most effective dosage of cetrorelix acetate to prevent a premature LH surge [151617]. Albano et al. [3] compared daily doses of 0.5, 0.25, and 0.1 mg of cetrorelix in women undergoing IVF. They found that dosages of both 0.5 and 0.25 mg prevented a LH surge, while a premature LH surge was observed in two of the seven patients who received a dose of 0.1 mg.
Similarly, a multicenter study was performed on 333 women who received six different dosages of ganirelix (0.0625, 0.125, 0.25, 0.5, 1.0, and 2.0 mg/0.5 mL) administered once daily by a subcutaneous injection. They found that serum concentrations of ganirelix increased in a linear manner, whereas serum LH and the increase of E2 fell with increasing ganirelix doses. During ganirelix treatment, serum LH concentrations ≥10 IU/mL were observed in the lowest dose groups, with incidences of 16% (0.0625 mg), 9% (0.125 mg), and 1.4% (0.25 mg). On the day of hCG administration, the number of follicles measuring ≥11, ≥15, and ≥17 mm was similar across the six dose groups, whereas serum E2 concentrations were highest in the 0.0625 mg group (1,475 pg/mL) and lowest in the 2 mg group (430 pg/mL) [4].
In the present study, the fertilization method in all cases was intracytoplasmic sperm injection (ICSI); a single fertilization method was used to avoid bias and fallacious conclusions. Although ICSI is more expensive than IVF, it was highly accepted by our study population and commonly requested. Subjects excluded from the study for financial reasons underwent IVF to complete their trial, but were not added to the population analysed in this study.
In the present study, reducing the GnRH-ant dosage from 0.25 to 0.125 mg was found to lead to the same clinical and ongoing pregnancy rates. This reduced dose of GnRH-ant was associated with a significant reduction in cost, from US $618.75 for the 0.25-mg protocol to US $499.34 for the 0.125-mg protocol (p=0.003). There were no reported cases of OHSS, as shown in Tables 2 and 3.
Huang et al. [18] compared a reduced cetrorelix dose (0.125 mg) to GnRH-a in 120 unselected women. They found that the primary and secondary outcomes were comparable in both groups. Moreover, a shorter duration of stimulation, a lower dosage of recombinant FSH, and a thinner endometrium on the day of triggering were all observed in the GnRH-ant group. They concluded that the reduced cetrorelix dose (0.125 mg) was effective for these unselected patients during IVF-embryo transfer.
Another study was performed in Asian women, as they weigh less than Caucasian women; therefore, the researchers suggested that the GnRH-ant dosage should be adjusted. In that series, it was concluded that a lower dose (0.2 mg daily dosage of cetrorelix) for Asians with a lower body weight (<50 kg) should be considered. They noted that 0.15 mg of cetrorelix daily was not suitable for LH suppression, and that the clinical pregnancy/implantation rates in the 0.2 mg group were higher than in the 0.15 mg group. They also noted that 0.2 mg of cetrorelix appeared to have a comparable pregnancy rate (around 30%) to that of 0.25 mg of cetrorelix. The LH surge risk for the patients who received the 0.2 mg daily dose was as low as those who received 0.25 mg [10].
A reduction of the cetrorelix dose by half in an ICSI antagonist protocol was not associated with any significant differences in the number of oocytes retrieved or in the pregnancy rate. Moreover, it was more economically feasible for patients in a low-resource country.
Acknowledgments
We want to thank all the nursing and statistics staff at the Egyptian Consultants' Center and the Qurrat Aien Center for their tremendously useful help and cooperation in data collection.
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.