T helper cell subsets and related cytokines in infertile women undergoing in vitro fertilization before and after seminal plasma exposure
Article information
Abstract
Objective
In vitro fertilization (IVF) is a well-known method for the treatment of infertility. The present study aimed to compare the differences between infertile women with successful and unsuccessful IVF outcomes regarding the expression of T helper (Th) cell transcription factors and a group of related cytokines before and after exposure to their husbands' seminal plasma.
Methods
This study was performed on 19 couples with unexplained infertility undergoing IVF treatment. Among the studied group, nine and 10 couples had successful and unsuccessful IVF outcomes, respectively. This study was carried out using real-time polymerase chain reaction.
Results
Before seminal plasma exposure, the expression levels of T-bet (p<0.007), interferon-γ (p=0.013), and tumor necrosis factor (TNF)-α (p=0.017) were higher in the infertile women with IVF failure than in those with successful IVF outcomes, while those of GATA3 (p<0.001), Foxp3 (p=0.001), and interleukin (IL)-35 (p<0.003) were lower. After seminal exposure, the expression of T-bet (p=0.02), Rorc (p<0.001), TNF-α (p=0.001), Foxp3 (p=0.02), and interferon-γ (p=0.001) increased in the unsuccessful IVF group, while the expression of Foxp3 (p=0.02), Rorc (p<0.001), IL-23 (p=0.04), IL-17 (p=0.02), IL-6 (p<0.001), transforming growth factor-β (p=0.01), and IL-35 (p<0.001) increased in the successful IVF group.
Conclusion
In summary, IVF failure was associated with imbalanced Th1/Th2/Th17/Treg responses. Moreover, our results show that seminal plasma might have a positive effect on IVF outcomes via changes in peripheral blood T cell subsets.
Introduction
Infertility has been defined as the failure to conceive successfully after 1 year of unprotected intercourse. This condition affects approximately 7%–15% of couples of reproductive age [1]. Many factors, such as endocrine and immunological factors, might predispose a couple to infertility. Some infertile couples are classified as having unexplained infertility because the reasons for their infertility remain unknown. Some evidence has been found to indicate that the immune system is involved in unexplained infertility [2].
In vitro fertilization (IVF) is widely used to treat infertility, but the outcomes of IVF are diverse and unpredictable. There is increasing evidence of the role of the maternal immune system in infertility [234]. Both humoral and cell-mediated immunity have been reported as possible mechanisms of implantation failure [56]. Regulation of the maternal immune response has also been found to be critical for implantation and the achievement of a successful pregnancy. In this regard, homeostasis between the type 1 helper T cell (Th1) and type 2 helper T cell (Th2) responses and Th1/Th2 polarization in implantation have been studied for many years. Recent studies have reported that fine-tuned balance and coordination among different subsets of T helper cells contributed to fertility and the success of pregnancy [7]. Distinct T cell subsets secrete various cytokines that play different roles in a complex regulatory pathway. Th1 cells produce inflammatory cytokines, such as interferon (IFN)-γ and IFN-α, thereby promoting cell-mediated immunity. In contrast, Th2 cells are involved in humoral immunity by producing interleukin (IL)-4, IL-5, and IL-13 [7].
For many years, the Th1/Th2 paradigm has been used as a framework for predicting pregnancy outcomes. Several studies have confirmed that successful pregnancy is associated with a predominant Th2-type immunity, while Th1-type immunity is associated with pregnancy-related disorders, such as recurrent pregnancy loss (RPL) [8]. After the discovery of newer T helper subsets, we now speak of the Th1/Th2/Th17/Treg paradigm, rather than the Th1/Th2 paradigm [7]. T helper 17 cell (Th17) is a novel subset of T cells that develop from naive CD4+ T cells and secrete IL-17A, IL-17F, and IL-22. Rorc is the major transcriptional factor for Th17 development in humans and mice [910]. Th17 cells are directly involved in chronic inflammatory processes by secreting IL-17, which recruits neutrophils to tissues through the induction of granulocyte colony-stimulating factor and IL-8 [11]. Few published studies have focused on the role of Th17 cells in women with RPL. The results of those studies showed that Th17 cells and their related cytokines, including IL-23 and IL-17, were elevated in women with RPL compared to those with normal pregnancies [12]. Increased numbers of IL-17+ T cells and a higher ratio of Th17 to CD4+/CD25+ regulatory T cells (Treg) have also been shown to be associated with pregnancy loss [13].
The main types of T cells generated in the periphery from naive CD4+ T cells under the influence of transforming growth factor (TGF)-β are Treg. Foxp3 has been identified as a major transcription factor for the generation and development of Treg [12]. In mice and humans, Treg increase during pregnancy and play a key role in protecting the fetus from the adverse effects of maternal immune responses against the semi-allograft fetus [14]. In line with the important role of Treg in pregnancy, several studies have shown that the number of Treg was lower in the peripheral blood or decidua of women with RPL [14].
The successful implantation and maintenance of a normal pregnancy requires cellular and molecular changes within the uterine environment during the preimplantation period [1516]. In reproduction, seminal plasma (SP) is considered to be means for the transport of spermatozoa to the cervix and uterus. In addition, previous studies have reported that SP provides a rich environment for successful implantation [17]. Several molecules with immunological activity have been shown to be present in SP, including TGF-β, IFN-γ, prostaglandin E2, tumor necrosis factor (TNF)-α, IL-1β, IL-6, IL-8, IL-10, and IL-12 [18]. These molecules are able to induce a classic inflammatory response within the female reproductive tract after insemination, which is necessary for successful implantation [19]. It has also been shown that semen can influence the production of certain cytokines, such as IL-10 [20]. Studies using mouse models have demonstrated that SP increased the regulatory T cell pool and induced tolerance to paternal alloantigens [2122]. The elevated synthesis of IL-1β, IL-6, and leukemia inhibitory factor by endometrial epithelial cells after culture with SP has also been document [23]. Overall, it seems that seminal fluid induces important immune reactions in the female reproductive tract that result in embryo development, implantation, and trophoblast invasion. Moreover, activated lymphocytes following insemination might mediate the maternal tolerance of the conceptus at the implantation site [24]. In this regard, some clinical evidence has confirmed that intercourse or artificial insemination during IVF cycles promotes embryo development and improves pregnancy rates [25]. Therefore, for a successful implantation and pregnancy, it is not only necessary for a sufficient number of spermatozoa to reach the oocyte in the oviduct, but also for the semen to regulate immune responses to ensure a successful outcome [252627].
The present study aimed to investigate peripheral blood Th1, Th2, Th17, and Treg subsets and their related cytokines in infertile women who were candidates for IVF. Considering the importance of semen in the development of immune responses during successful implantation and pregnancy, another goal of the study was to investigate the effects of human semen on the production and differentiation of T cell subsets and their related cytokines in these women.
Methods
1. Study population
This study was performed in 19 infertile couples who were candidates for IVF. Within this sample, 10 and nine couples had failed (unsuccessful) and successful IVF outcomes, respectively. A successful outcome was defined as a positive pregnancy test, while an unsuccessful outcome was defined as a negative human chorionic gonadotropin test after embryo transfer. It should be noted that all the couples had unexplained infertility and no history of recurrent miscarriage, autoimmune diseases, or immunodeficiency. Infertility was diagnosed by the same gynecologist and infertility specialist in couples who had engaged in regular unprotected intercourse for at least 1 year. The women were all undergoing IVF treatment at the IVF Center at Mother and Child Hospital, Shiraz University of Medical Sciences, Shiraz, Iran. The women did not engage in sexual intercourse for at least 2 weeks before IVF, and were not exposed to SP before or during the IVF procedure. Informed consent was obtained from the participants, and the study was approved by the Local Ethics Committee of Shiraz University of Medical Sciences for the use of blood samples and seminal fluid (No. 93-6979).
2. Semen collection and processing
SP was obtained from all 19 partners of the infertile women. First, the semen samples were collected by masturbation using sterile containers. All semen samples had a normal sperm count, motility, and morphology according to the 2010 World Health Organization semen analysis criteria (5th edition) and were free of lymphoid cells (checked by light microscopy). The semen samples were allowed to liquefy for 30 minutes, and the SP was separated by centrifugation of the ejaculates at 1,200 rpm at room temperature for 10 minutes. The second round of centrifugation was performed at 10,000 rpm for 12 minutes to completely remove all the spermatozoa. Finally, SP samples were collected from the supernatants and stored at −20℃ until co-culture with peripheral blood mononuclear cells (PBMCs).
3. Isolation and culture of PBMCs
Fresh PBMCs were isolated from the infertile women using Ficoll density gradient centrifugation. Mononuclear cells were suspended in RPMI 1640 medium (Shellmax, Taizhou, China) supplemented with 10% fetal bovine serum (Shellmax) and 1% penicillin-streptomycin (Invitrogen, Carlsbad, CA, USA). Subsequently, 1×106 separated PBMCs were stored in liquid nitrogen in order to extract the total RNA for the analysis of gene expression using real-time polymerase chain reaction (RT-PCR). Moreover, 1×106 separated PBMCs were used for co-culture with SP. At first, the separated cells were supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin in RPMI medium and were then cultured with or without 0.33% SP for 24, 48, and 72 hours at 37℃ and 5% CO2. At each time point, the viability of the cells was assessed by trypan blue staining. Wells with >90% viability were harvested and stored in liquid nitrogen until the extraction of total RNA.
4. RNA extraction
RNA was extracted from a pellet of approximately 1×106 PBMCs using a total RNA extraction kit (Jena Bioscience, Jena, Germany) following the manufacturer's instructions. The final RNA pellet was dissolved in 25 µL of diethyl pyrocarbonate (DEPC)-treated water. The concentration of the extracted RNA was determined using a Nano Drop instrument (NanoDrop 2000c; Thermo Scientific, Waltham, MA, USA). Agarose gel electrophoresis was used to check the purity and quality of the extracted mRNA. All extracted RNA was immediately converted to cDNA after extraction.
5. cDNA synthesis
cDNA was synthesized from 700 ng of total RNA using an easy cDNA synthesis kit (Cinnagen, Tehran, Iran) based on the manufacturer's instructions. The final volume of the cDNA synthesis reaction was 20 µL, including 2 µL of RNA, 2 µL of a random hexamer and oligo-dT, 6 µL of DEPC-treated water, and 10 µL of ×2 reverse transcriptase reaction buffer. The synthesis tube was then incubated at 65℃ for 5 minutes, 25℃ for 10 minutes, 47℃ for 60 minutes, and 70℃ for 10 minutes. The quality of all synthetic cDNA was checked by PCR.
6. Quantitative RT-PCR
The mRNA levels of all cytokines and T cell transcription factors (TNF-α, IFN-γ, IL-17A, IL-23, IL-31, IL-6, IL-35, TGF-β, Rorc, Foxp3, GATA3, and T-bet) were measured by means of RT-PCR using the SYBR Green method (GeNet Bio, Daejeon, Korea) in a StepOne Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). Briefly, 20 pmol of each primer (Table 1), 2 µL of target cDNA (selected after checking and normalizing the CT values), and 10 µL of the SYBR Green cocktail were used for amplification. The final volume of each reaction was 20 µL. The quantitative PCR (Q-PCR) reactions were carried out as follows: initial denaturation at 95℃ for 30 seconds followed by 40 cycles of denaturation at 95℃ for 5 seconds and annealing and amplification at 60℃ for 34 seconds. The comparative CT and fold differentiation (2−ΔΔCT) methods were used to quantify target gene expression. Furthermore, the calibrator sample and 18s rRNA (a control housekeeping gene) were used to minimize the variation and to normalize the Q-PCR, respectively.
7. Statistical analysis
The Mann-Whitney test was used for the quantitative analysis of the Q-PCR results using GraphPad Prism ver. 5.0 (GraphPad Software Inc., San Diego, CA, USA), and p-values less than 0.05 were considered to indicate statistical significance. In addition, ΔCT values were used to compare gene expression in the cases and controls, and 2−ΔΔCT was used for relative gene expression analysis.
Results
1. Differences between the women with successful and unsuccessful IVF outcomes regarding the expression of T cell transcription factors
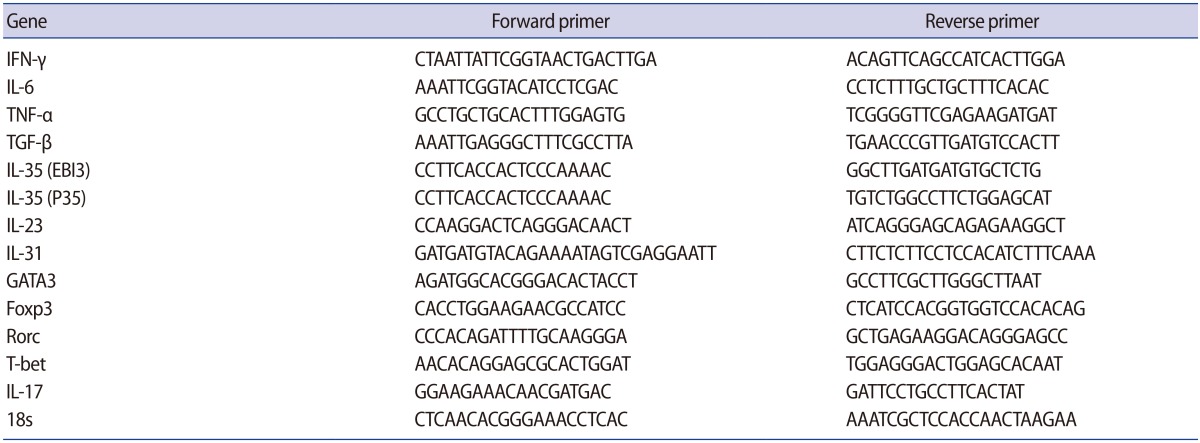
The expression of the T-bet transcription factor was significantly higher in the women with IVF failure than in those with successful IVF outcomes (p=0.007). However, the expression of the GATA3 and Foxp3 transcription factors was significantly lower in the women with IVF failure than in those with successful IVF outcomes (p<0.001 and p=0.001, respectively) (Figure 1). No significant differences were found between the two groups regarding the expression of the Rorc transcription factor.
2. Differences between the women with successful and unsuccessful IVF outcomes regarding the expressions of cytokines associated with Th1, Th2, Th17, and Treg subsets
As shown in Figure 2, the expression of IFN-γ (p=0.013) and TNF-α (p=0.017) was upregulated in the women with IVF failure in comparison to the successful IVF group. However, the expression of both chains of the IL-35 cytokine was downregulated in the women with IVF failure compared to the successful IVF group (p=0.035 and p=0.03 for the EBI3 and P35 subunits, respectively). Nevertheless, no significant differences were observed between the two groups regarding other cytokines (p=0.7 for both IL-23 and IL-17, p=0.09 for both IL-6 and TGF-β, and p=0.4 for IL-31).
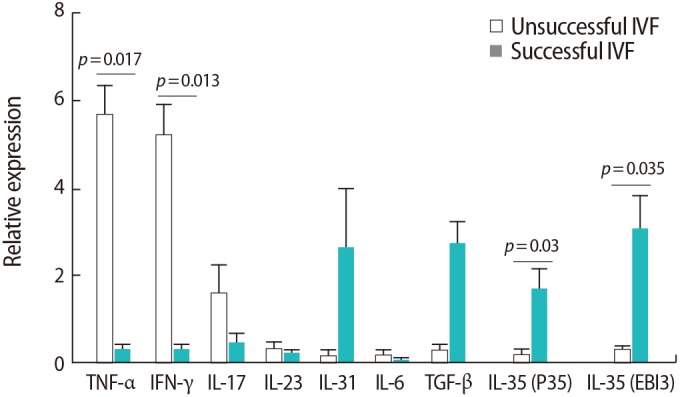
Comparison of the relative expression levels of the studied cytokines in peripheral blood samples from infertile women with successful (n=9) and unsuccessful (n=10) in vitro fertilization (IVF) outcomes. The data were analyzed by the Mann-Whitney test. TNF, tumor necrosis factor; IFN, interferon; IL, interleukin; TGF, transforming growth factor.
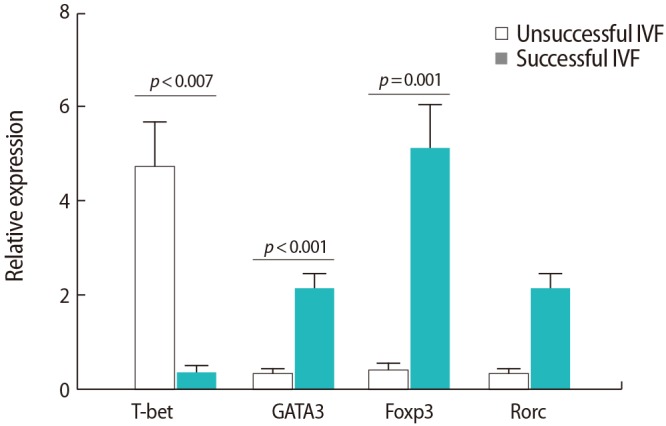
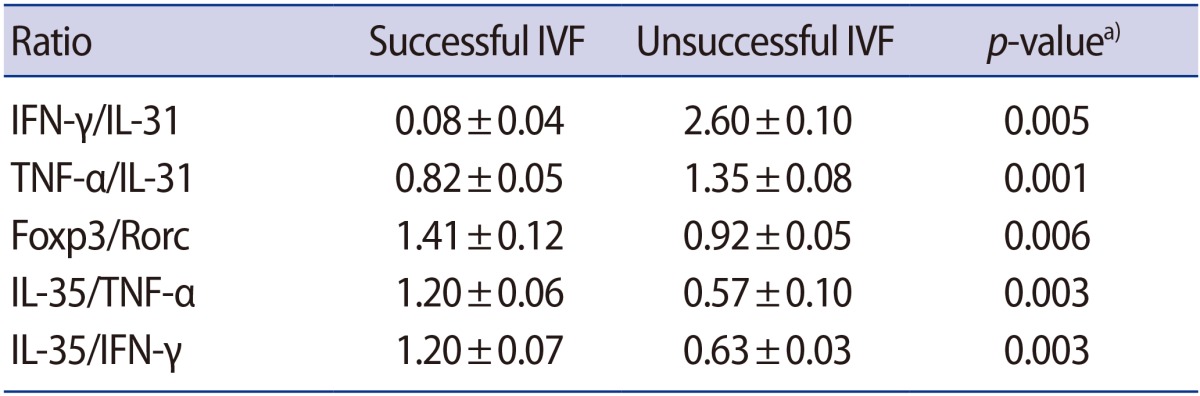
The ratio of the expression of cytokines to the expression of transcription factors was also investigated. The significant results are presented in Table 2. While the ratios of T-bet to GATA3, TNF-α to IL-31, TNF-α to GATA3, and IFN-γ to GATA3 were all higher in women with IVF failure than in the successful IVF group, the ratios of Foxp3 to Rorc, Foxp3 to IL-17, IL-35 to TNF-α, IL-35 to IFN-γ, and IL-35 to IL-17 were significantly lower in the IVF failure group.
3. Difference in the expression of T cell transcription factors before and after SP exposure
In both groups of women, no significant changes were seen in GATA3 expression after SP exposure. However, both groups expressed more GATA3 after SP exposure. T-bet expression significantly increased after SP exposure only in women with unsuccessful IVF outcomes (p=0.02). The expression of the Rorc transcription factor was also upregulated after SP exposure in both groups (p<0.001). Foxp3 expression also showed significant upregulation after SP exposure in both groups (p=0.02) (Figure 3).
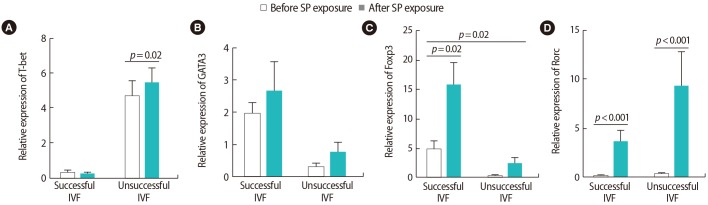
Comparison of the relative expression levels of T cell transcription factors of (A) T-bet, (B) GATA3, (C) Foxp3, (D) Rorc before and after seminal plasma (SP) exposure in peripheral blood samples from infertile women with successful (n=9) and unsuccessful (n=10) in vitro fertilization (IVF) outcomes. The data were analyzed by the Mann-Whitney test, and p-values <0.05 were considered to indicate statistical significance.
4. Differences in the expression of cytokines associated with Th1, Th2, Th17, and Treg responses after SP exposure
Our investigation of cytokine expression indicated that after SP exposure, the expression of TNF-α was upregulated in the women with unsuccessful IVF outcomes (p=0.001). In the women with successful outcomes, however, the expression of IL-17 (p=0.02), IL-23 (p=0.04), and IL-6 (p<0.001) significantly increased. After SP exposure, no significant changes were observed in IL-31 expression in either group (Figure 4). In the women with successful outcomes, the expression of all Treg-associated cytokines (i.e., TGF-β [p=0.003] and IL-35 [p<0.001 for P35 and p<0.001 for EBI3]), showed significant upregulation after SP exposure (Figure 5).
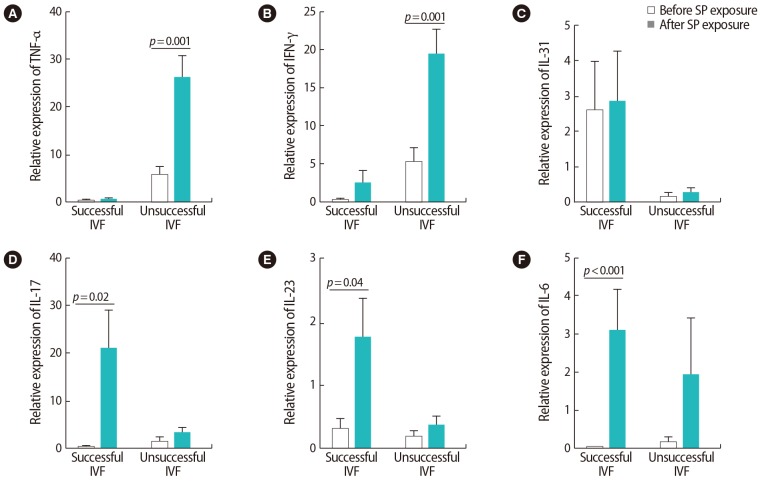
Comparison of the relative expression levels of (A) tumor necrosis factor (TNF)-α, (B) interferon (IFN)-γ, (C) interleukin (IL)-31, (D) IL-17, (E) IL-23, (F) IL-6 before and after seminal plasma (SP) exposure in peripheral blood samples from infertile women with successful and unsuccessful in vitro fertilization (IVF) outcomes. The data were analyzed by the Mann-Whitney test, and p-values <0.05 were considered to indicate jstatistical significance.
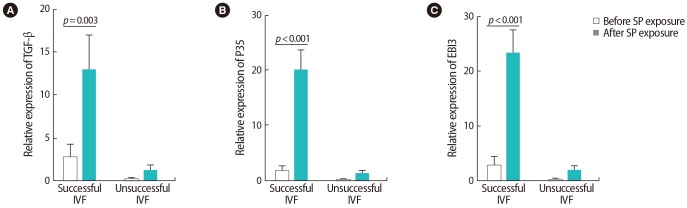
Comparison of the relative expression levels of (A) transforming growth factor (TGF)-β, (B, C) interleukin (IL)-35 (P35 and EBI3) before and after seminal plasma (SP) exposure in peripheral blood samples from infertile women with successful and unsuccessful in vitro fertilization (IVF) outcomes. The data were analyzed by the Mann-Whitney test, and p-values <0.05 were considered to indicate statistical significance.
The results of the comparison between successful and unsuccessful IVF groups regarding the ratio of the expression of cytokines after SP exposure are presented in Table 3. The ratios of IFN-γ to IL-31 and TNF-α to IL-31 were significantly higher (p=0.005 and p=0.001, respectively) in the women with IVF failure than in those with successful IVF outcomes, while the ratios of Foxp3 to Rorc, IL-35 to TNF-α, and IL-35 to IFN-γ were significantly lower (p=0.006, p=0.003, and p=0.003, respectively) (Table 3). Moreover, a comparison of the relative expression of transcription factors and their related cytokines before and after SP exposure only found two significant changes: the ratios of IFN-γ to IL-31 and Foxp3 to Rorc were upregulated in the unsuccessful and successful groups, respectively (p=0.005 for both comparisons, not shown in a table).
5. Correlations between T cell transcription factors and their associated cytokines after SP exposure
After SP exposure, a negative correlation was found between the expression of Foxp3 and Rorc (r=−0.65, p=0.038) (Figure 6A), IL-35 (EBI3) and TNF-α (r=−0.75, p=0.072) (Figure 6B), and TGF-β and T-bet (r=−0.59, p=0.006) (Figure 6C) in the women with successful IVF outcomes. In contrast, in the women with unsuccessful IVF outcomes, the expression of the Rorc transcription factor was positively correlated with TNF-α (r=0.75, p=0.01) (Figure 7A) and the expression of T-bet was correlated to IFN-γ (r=0.81, p=0.005) (Figure 7B) before SP exposure. After SP exposure, however, the expression of IFN-γ showed a positive correlation with TNF-α (r=0.69, p=0.03) (Figure 8A) and the expression of IL-17 was correlated to TNF-α (r=0.65, p=0.041) (Figure 8B) and IFN-γ (r=0.75, p=0.02) (Figure 8C) in the women with unsuccessful IVF outcomes.
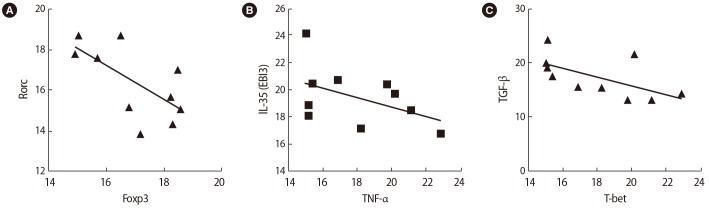
Correlations between the expression of T cell transcription factors and their associated cytokines in infertile women with successful in vitro fertilization outcomes before seminal plasma exposure: (A) Rorc and Foxp3, (B) interleukin (IL)-35 (EBI3) and tumor necrosis factor (TNF)-α, (C) transforming growth factor (TGF)-β and T-bet.
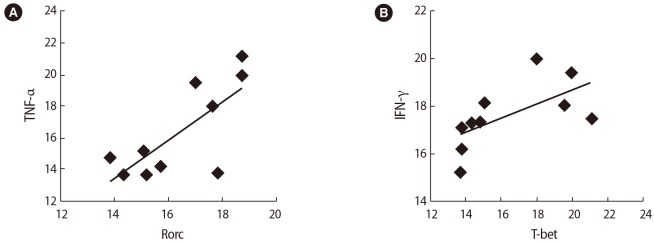
Correlations between the expression of T cell transcription factors and their associated cytokines in infertile women with unsuccessful in vitro fertilization outcomes before seminal plasma exposure: (A) tumor necrosis factor (TNF-α) and Rorc, (B) interferon (IFN)-γ and T-bet.
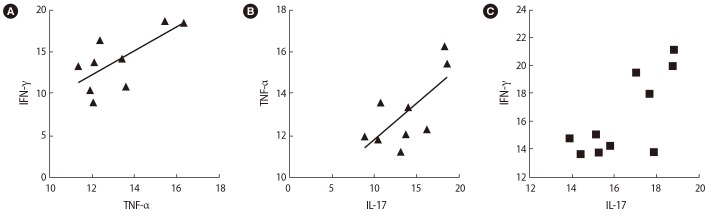
Correlations between the expression of T cell transcription factors and their associated cytokines in infertile women with unsuccessful in vitro fertilization outcomes after seminal plasma exposure: (A) interferon (IFN)-γ and tumor necrosis factor (TNF-α) (B) TNF-α and interleukin (IL)-17, (C) IFN-γ and IL-17.
Discussion
This study compared the expression of the transcription factors of Th cell subsets and their related cytokines in infertile women with successful and unsuccessful IVF outcomes before and after SP exposure. Previous studies have documented that a group of infertility patterns might be associated with inappropriate activity of the immune system. Indeed, several studies have indicated that effector mechanisms of cellular immunity played an important role in the etiology of unexplained infertility [234]. Moreover, it is generally agreed that pregnancy is associated with a predominance of Th2-type immunity, while an increased Th1 immune response is associated with RPL and multiple implantation failures [28293031]. In line with previous studies, the results of the present research showed a Th1 bias regarding immune responses in the infertile women with IVF failure. A dominant Th1 response in women with IVF failure was also demonstrated by the increased expression of T-bet and its associated Th1 cytokines IFN-γ and TNF-α. In contrast, Th2-dominant responses in the infertile women with successful IVF outcomes were associated with the upregulation of GATA3 expression. Moreover, in agreement with previous findings, our results showed that infertile women with IVF failure had higher ratios of T-bet to GATA3, TNF-α to GATA3, IFN-γ to GATA3, and TNF-α to IL-31 than the successful IVF group. Therefore, an adequate balance of Th1 and Th2 immunity that is slightly shifted towards Th2-type immunity may be effective for achieving a successful IVF outcome. However, overstimulation of the Th1 or Th2 immune response may reduce the likelihood of a successful IVF outcome. In recent years, the important role of the Th17/Treg subsets in pregnancy outcomes has been highlighted and, as a result, the Th1/Th2 paradigm has been expanded toward the Th1/Th2/Th17/Treg paradigm. Th17 and Treg cells have opposite effects, as Th17 plays an important role in the inflammatory immune response, but Treg cells are a potent suppressor of inflammation [1432]. Both Treg and Th17 cells are involved in the establishment and maintenance of pregnancy as regulator and effector cells, respectively. Several studies have suggested that an imbalance between regulatory and effector cells might lead to implantation failure and many other pregnancy-related disorders [7]. Treg have recently been considered as key players in protecting the conceptus from the adverse effects of alloreactive responses that are mediated by the mother's immune system [33]. Furthermore, the number of Treg has been reported to diminish in spontaneous abortion compared to elective abortion [34]. The results of the present work indicated that the Treg transcription factor (Foxp3) and IL-35 expression were significantly higher in the women with successful IVF outcomes than in the IVF failure group. IL-35 is an anti-inflammatory cytokine that belongs to the IL-12 family and is mainly produced by Treg. IL-35 exerts a suppressive activity on the immune system through the expansion of Treg and the inhibition of Th17 cell development [3536]. A few studies have reported that IL-35 significantly increased in normal pregnancies and decreased in recurrent spontaneous abortion [37]. Moreover, the ratio of IL-35 to IL-17 has been reported to be lower in infertile women than in healthy ones [3839]. As in other published studies, our results showed an increase in the Treg to Th1 ratio (IL-35 to INF-γ and IL-35 to TNF-α) and negative correlations between IL-35 and TNF-α, T-bet, and TGF-β in the women with successful IVF outcomes compared to those with IVF failure. This result underscores the importance of the regulation of immune responses, as well as inflammatory responses, at the time of fertilization and implantation for the success of IVF. Supporting this hypothesis, our results indicated that in women with unsuccessful IVF outcomes, inflammatory responses were upregulated, while regulatory responses were diminished. Although our results showed no significant differences between the two studied groups regarding IL-17 production, the ratios of Treg/Th17 transcription factors to their related cytokines (Foxp3 to Rorc, Foxp3 to IL-17, and IL-35 to IL-17) were higher in the women with successful outcomes. Furthermore, a negative correlation was detected between Foxp3 and Rorc in the women with successful IVF outcomes. These results show that the inflammatory response by Th17 must be controlled by Treg cells in order to achieve successful fertilization and implantation.
In the second part of this study, for the first time, the effect of SP on the expression of T cell transcription factors and their related cytokines was investigated in infertile women with successful and unsuccessful IVF outcomes. In the successful IVF group, SP exposure increased the inflammatory response. In this group, along with an increase in inflammation, regulatory responses were also upregulated to control inflammation. In the women with IVF failure, although the inflammatory responses increased, the regulatory responses did not increase in parallel to control the adverse effects of inflammation. Thus, it seems that women with IVF failure are not able to control the inflammatory response and that semen exposure is not able to compensate for this problem. In line with this hypothesis, the findings of the current study demonstrated an increase in the expressions of Rorc, T-bet, IFN-γ, and TNF-α, but no overexpression of regulatory T cell cytokines, after SP exposure in patients with IVF failure. Therefore, in the unsuccessful group, SP might have exacerbated inflammation by increasing the expansion of Th1 and Th17 cells. On this basis, it can be concluded that women with IVF failure might have a defect in the induction of their Treg that cannot be compensated for by SP exposure. In a recently published paper, Robertson et al. [40] reviewed the effects of SP on the cervix, endometrium, immune responses, and outcomes of assisted reproductive technology and IVF. Seminal fluid was reported to play a role in embryo development, implantation, and trophoblast invasion [23]. Moreover, several previous studies have revealed the role of SP in feto-maternal tolerance and the generation of Treg [4142]. Among the many proposed effects of semen, the induction of proinflammatory cytokines and chemokines, which are important for implantation, is well known [43]. In line with this important effect of SP, the results of the present study indicated that SP might increase the expression of inflammatory cytokines and inflammatory T cell subsets. In both the successful and unsuccessful groups, SP exposure elevated the expression of inflammatory cytokines. Hence, it may be concluded that SP affects the success of implantation by priming the mother to accept the fetus. Furthermore, seminal fluid contains several potent immune suppressor agents that might induce the expansion of inducible Treg cells. Indeed, the most important pathway mediated by SP is the activation and expansion of maternal Treg [41].
Consistent with previous studies, the findings of the present study indicate that SP might induce Treg and their related cytokines. Interestingly, this effect was only observed in the women with successful IVF outcomes. In the women with unsuccessful outcomes, however, SP exposure increased Treg cytokines. Nonetheless, this increase was not significant and was not sufficient to control the inflammation. This discrepancy may have been due to a difference between the successful and unsuccessful groups regarding the composition of semen, including semen cytokines or Treg, and this possibility should be investigated further. Moreover, SP exposure might reduce the risk of complications of pregnancy, such as pre-eclampsia and effects on the offspring's metabolic phenotype [44]. Although no paper has been previously published with which we could compare our results directly, some studies have suggested that women with pregnancy disorders, such as RPL, had a proinflammatory tendency via Th1 and Th17 immunity and a decrease in immune regulatory function by Foxp3+ Treg [41]. In summary, the results of the present study indicate that IVF failure was associated with imbalanced Th1/Th2/Th17/Treg responses. The results also showed that SP might have a positive effect on IVF outcomes via changes in peripheral blood T cell subsets.
Acknowledgments
The authors would like to thank Ms. Afsaneh Keivanshekouh at the Research Improvement Center of Shiraz University of Medical Sciences for improving the use of English in the manuscript.
Notes
This study was extracted from the MSc thesis written by Marziyeh Azad and financially supported by the Shiraz University of Medical Sciences, Iran (No. 93-6979).
Conflict of interest: No potential conflict of interest relevant to this article was reported.