Subretinal transplantation of putative retinal pigment epithelial cells derived from human embryonic stem cells in rat retinal degeneration model
Article information
Abstract
Objective
To differentiate the human embryonic stem cells (hESCs) into the retinal pigment epithelium (RPE) in the defined culture condition and determine its therapeutic potential for the treatment of retinal degenerative diseases.
Methods
The embryoid bodies were formed from hESCs and attached on the matrigel coated culture dishes. The neural structures consisting neural precursors were selected and expanded to form rosette structures. The mechanically isolated neural rosettes were differentiated into pigmented cells in the media comprised of N2 and B27. Expression profiles of markers related to RPE development were analyzed by reverse transcription-polymerase chain reaction and immunostaining. Dissociated putative RPE cells (105 cells/5 µL) were transplanted into the subretinal space of rat retinal degeneration model induced by intravenous sodium iodate injection. Animals were sacrificed at 1, 2, and 4 weeks after transplantation, and immnohistochemistry study was performed to verify the survival of the transplanted cells.
Results
The putative RPE cells derived from hESC showed characteristics of the human RPE cells morphologically and expressed molecular markers and associated with RPE fate. Grafted RPE cells were found to survive in the subretinal space up to 4 weeks after transplantation, and the expression of RPE markers was confirmed with immunohistochemistry.
Conclusion
Transplanted RPE cells derived from hESC in the defined culture condition successfully survived and migrated within subretinal space of rat retinal degeneration model. These results support the feasibility of the hESC derived RPE cells for cell-based therapies for retinal degenerative disease.
Introduction
The retina is part of the central nervous system and plays the most important role in the visual pathway. In the retinal degenerative diseases such as age-related macular degeneration and genetic retinal diseases like Stargardt disease, dysfunction and loss of retinal pigment epithelium (RPE) are major pathologic changes leading to visual loss [1]. The RPE is composed of a monolayer of pigmented cells that lies adjacent to photoreceptor outer segments and plays a crucial role in retinal homeostasis. The RPE cells are critical for photoreceptor survival, including nutrient and ion transport, light absorption, recycling of retina, and formation of blood-retinal barrier [2]. Consequently, RPE dysfunction leads to photoreceptor loss and subsequent central visual loss. However, there is no radical therapeutic modality to recover damaged RPE or photoreceptor cells in retinal degenerative disease. To restore vision in these patients, one promising therapeutic strategy could be cell-based transplantation therapies to replace damaged retinal cells with functioning cells [3-8].
Stem cells are ideal candidate donor source because of their capacity to self-renew and generate multiple differentiated cell types. Unlike tissue stem cells, human embryonic stem cells (hESC) retain unlimited self-renewal potential and are expected to alleviate the problem of donor cell shortage for cell-replacement therapy [9]. Although photoreceptor differentiation from embryonic stem cell is crucial for the cell-based therapy of vision restoration, successful differentiation into functional photoreceptor has not yet been reported. On the other hand, several investigators have reported RPE differentiation from hESCs [6,10-16]. There is increasing evidence to suggest that RPE cells derived from hESCs are more akin to RPE cells than cell lines originally created from human RPE tissue, when characterized in terms of morphology, gene expression, and immunohistochemical profile [6,10-12].
Recently we developed a new protocol to differentiate the hESC (SNUhES3) into putative RPE cells in the defined culture condition and confirmed the characteristics of RPE differentiation. In addition, we performed the subretinal transplantation of the differentiated RPE cells in a rat retinal degeneration model induced by sodium iodate and investigated the survival and integration of the transplanted cells.
Methods
1. Cell culture and derivation of putative RPE cells
The hESC, SNUhES3 was maintained as described previously [17]. Undifferentiated hESC colonies were detached mechanically and cultured in a bacterial dish for 7 days to form embryoid body (EB). For neural differentiation, cultured EBs (5 day-old) were attached on the matrigel-coated culture dishes and cultured to select the neural structures consisting neural precursors in media supplemented with 0.5% N2 for 5 days. The selected neural precursors were expanded to form rosette structures in the media with basic fibroblast growth factor (bFGF; #13256-029; Invitrogen, Carlsbad, CA, USA) and 1% N2 for seven to ten days. These rosettes structures were isolated mechanically and reattached onto matrigel-coated culture dishes. To facilitate the differentiation of reattached cells toward RPE fate, bFGF was replaced by B27 (2%) from the expansion medium for RPE differentiation and expansion. The mechanically isolated rosettes were differentiated to pigmented cells, which were presumed to be RPE cells.
2. Characterization of putative RPE cells
1) Immunocytochemistry
Pigmented cells were washed with phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde. After blocking overnight in 3% bovine serum albumin solution in PBS containing 0.1% Triton ×100, samples were incubated with primary antibodies overnight. Rabbit anti-tight junction protein ZO-1 (1:200, Zymed, South San Francisco CA, USA) and mouse anti-microphthalmia-associated transcription factor Mitf (1:200, Abcam, Cambridge, CB, UK) were primary antibodies used for immunocytochemistry. Normal rabbit IgG (1:200, Zymed) or mouse IgG (1:200, Zymed) were used as negative control antibodies. To detect antibodies, Alexa Fluor 488 donkey anti-mouse and anti-rabbit IgG (1:200, Molecular Probes, Eugene, OR, USA) and Alexa Fluor 594 donkey anti-mouse and anti-rabbit IgG (1:200, Molecular Probes) were used, and the cell nuclei were counterstained with 4'6-diamidino-2-phenylindole (DAPI; 10 µg/mL, Sigma-Aldrich, St. Louis, MO, USA).
2) Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was isolated from hESCs, EBs, putative RPE cells from mechanically isolated rosettes at day 5, 41 and 74 using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA). cDNA synthesis was conducted using AMV RT and oligo-dT as a primer according to the manufacturer's instructions (AccuPower RT PreMix; Bioneer, Daejeon, Korea). PCR amplification was performed with Taq Polymerase (Hipi Plus 5x PCR Premix; Elpis biotech, Daejeon, Korea) using a standard procedure. Values for each gene were normalized to expression levels of glyceraldehydes-3-phosphate dehydrogenase. PCR conditions were optimized, and a linear amplification range was determined for each pair of primers by varying the annealing temperature and cycle numbers. Primers used for the reactions were Pax6, Mitf, bestrophin, and RPE65. The sequences of the primers are available on request.
3. Subretinal implantation of putative RPE cells in sodium iodate induced rat retinal degeneration model
A total of nine 6-week-old Sprague Dawley rats were used. The animals were treated according to the regulations in the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and the study was approved by the IACUC of Seoul National University Hospital. The animals received a single intravenous injection of sterile 1% NaIO3 diluted in saline through a caudal vein at a dose of 50 mg/kg. Four days after administration, the animals were anesthetized with intramuscular injection of tiletamine/zolazepam and xylazine. The pupils were dilated with tropicamide 0.5% and phenylephrine hydrochloride (HCl) 2.5% eye drops, and topical anesthetic eye drops of proparacine HCl 0.5% were administered. Before implantation, putative RPE cells were incubated with DAPI (10 µg/mL) for 30 minutes, washed several times to remove DAPI in the media, and then dissociated by incubation for 5 minutes in 0.05% trypsin/0.1% ethylenediaminetetraacetic acid at 37℃. Under visualization with an operating microscope, dissociated putative RPE cells (105 cells/5 µL) were injected into the dorsal subretinal space via a transscleral approach using a 33-gauge needle attached to a Hamilton syringe. Immediately after injection, the fundus was examined and any animals with massive subretinal hemorrhage or vitreous hemorrhage were removed from the study. Cyclosporin A (210 mg/L, Cipol-N; Chong Kun Dang, Seoul, Korea) was administered into the drinking water from 1 day before transplantation until enucleation.
Three animals were sacrificed at each of one, two, and four weeks after putative RPE cell transplantation. After enucleation, the eyes were rapidly frozen in embedding compound (FSC 22; Leica Microsystems, IL, USA). Cryostat sections were washed with PBS and incubated in 3% bovine serum albumin solution in PBS containing 0.1% Triton ×100, followed by an overnight incubation in primary antibodies. Anti-ZO-1, anti-RPE65, and anti-Bestrophin antibodies were used for immunohistochemistry. Alexa Fluor 488 donkey anti-mouse IgG (1:200) and Alexa Fluor 594 donkey anti-rabbit igG (1:200) were used to detect the primary antibodies.
Results
1. Characterization of putative RPE cells
On light microscopic examination, differentiated cells from rosette showed hexagonal shape with monolayer structure on the culture dish, which is typical characteristic of the RPE cells. Figure 1A shows phase-contrast image of pigmented cells transferred on a cover glass on day 24 when the cells were cultured in N2 and B27 for RPE differentiation. During the differentiation process of pigmented cells from rosettes, expression profiles of markers related to development and cellular function of RPE were analyzed by RT-PCR and immunocytochemistry.
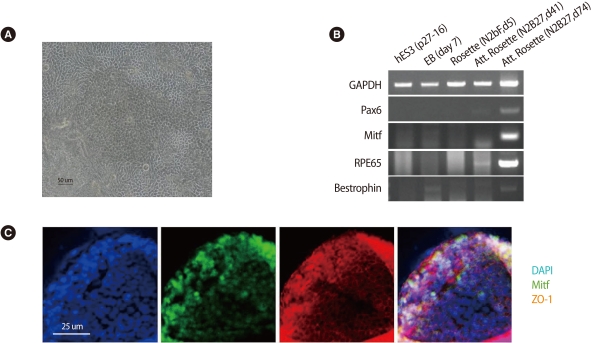
Confirmation of the putative retinal pigment epithelial cells (RPE cells) differentiated from human embryonic stem cell. (A) Pigmented cells transferred on a cover glass on day 24 formed a monolayer structure and showed hexagonal shape on phase-contrast image. (B) Expression of the RPE molecular markers detected by reverse transcription-polymerase chain reaction. (C) Immunostaining showed the expression of Mitf (early RPE cell marker) and ZO-1 (RPE associated tight junction protein). Scale bars: (A) 50 µm; (C) 25 µm. GAPDH, glyceraldehydes-3-phosphate dehydrogenase; DAPI, 4'6-diamidino-2-phenylindole.
RT-PCR showed the mRNA expression of Pax6 (early neural marker), Mitf (transcriptional factor in melanogenesis), bestrophin (RPE specific protein located in basolateral plasma membrane), and RPE65 (retinoid recycling) in the putative RPE cells from rosettes (Figure 1B). In immunostaining, polygonal pigmented cells from rosettes which had been maintained as adherent culture for 45 days expressed both the transcription factor Mitf and the RPE-associated tight junction protein ZO-1 (Figure 1C). Control antibody showed no staining (data not shown).
2. Survival and localization of the putative RPE cells after subretinal implantation in a rat retinal degeneration model
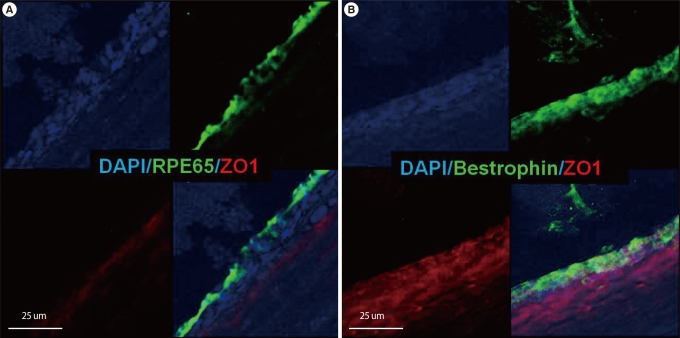
Hematoxylin and eosin staining of retinal section from sodium iodate injected rat enucleated one week after implantation showed transscleral injection site, surrounding retinal detachment, and cells with layer strucutre in the subretinal space (Figure 2). Subretinal cells were only visible posterior to the puncture site and were thought to be implanted putative RPE cells. The survival and the localization of the transplanted cells were confirmed by immunohistochemistry. Confocal microscopy showed the integration of DAPI-labeled cells in the subretinal space. They formed a layer and expressed mature RPE markers including bestrophin, RPE65, and ZO-1 at 1, 2, and 4 weeks after cell transplantation (Figure 3).
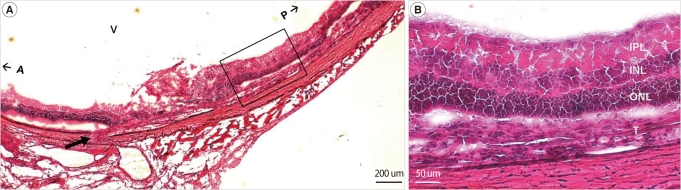
Retinal sections obtained one week after transplantation (H&E stain). (A) Transplanted cells in subretinal space between outer nuclear layer (ONL) and degenerative retinal pigment epithelium (RPE) was visible posterior to the trans-scleral puncture site (arrow). (B) High-power image of outlined area in Figure 2A. Scale bars: (A) 200 µm; (B) 50 µm. V, vitreous cavity; A, anterior; P, posterior; IPL, internal plexiform layer; INL, internal nuclear layer; ONL, outer nuclear layer; T, transplanted putative retinal pigment epithelium cells.
Discussion
A major issue in any cell-based therapy is provision of a cell source that is readily available, safe, and can be developed commercially in large-scale production. On those points, there are numerous advantages of using hESC-derived cells as a source for clinical studies. In addition, hESCs have high plasticity and migrating abilities compared to adult stem cells. In other regions of the central nervous system, the transplantation of neurons derived from ESC has led to some promising results. Dopaminergic neurons derived from mouse, monkey, and human ESC have been shown to integrate into the brain after transplantation and partially restore function in animal models of Parkinson's disease [18-22]. Although hESCs have many advantages as mentioned above, they have some limitations at the same time, including ethical issues and teratoma risks. However, the chief problem is that we are still struggling to understand the developmental cues that differentiate hESCs into the specific retinal cell types required for repair of damaged tissues.
It is worth noting that photoreceptor replacement is theoretically more straightforward than other retinal neurons because it is a sensory neuron connected in only one direction and does not require complex dendritic synapses to generate afferent inputs. However, evidence is still lacking for producing fully functional photoreceptor cells needed for retinal repair, although researchers have made progress in understanding the developmental stimuli that derive retinal neurons and photoreceptor progenitors from mouse or human ESCs in vitro [23]. Instead, RPE cells derived from hESCs would be another candidate for transplantation. Since Klimanskaya et al. [11] first reported the differentiation of RPE cells from hESC, several reports have described the potential of hESCs to differentiate into RPE cells in vitro [10-14]. In addition, subretinal transplantation of hESC-derived RPE cells in animal model preserved visual function and photoreceptor integrity [6,15,16]. As RPE cells do not require synaptic reconnection, RPE transplantation may be less complex than photoreceptors and retinal neurons.
The putative RPE cells derived from the hESC line SNUhES3 was well-characterized here and satisfied many of the known criteria of RPE cells, including morphology, cellular pigmentation, and expression of markers associated with RPE fate. These properties were similar to those observed in cultured hESC-derived RPE, although we did not included functional analysis of hESC-derived RPE cells like in vitro phagocytosis assays [6,10-12]. As an exploration for another protocol to differentiate RPE cells, further investigation is in progress by our group to generate RPE cells from hESC-derived spherical neural masses (SNMs) which we derived from EBs as described previously [18,24].
For implantation of cells, we used trans-scleral subretinal injection in this study. This method places the grafted cells closer to their intended location than other approaches, but it is more complex and technically demanding. On the other hand, intravitreal injection is less invasive and easier, but grafted cells must migrate from the vitreous cavity to the outer retina. Although there are conflicting results on the superiority of transplantation methods, subretinal injection is thought to be more physiologic if it were not for severe complication like intraocular hemorrhage or massive retinal detachment and is preferred by more researchers than intravitreal injection [25-27].
There are some points to improve the feasibility of the clinical application of cell-based therapy in retinal degenerative diseases. The assessment of photoreceptor rescue or visual function recovery after hESC-RPE cell transplantation could be achievable with electrophysiological evaluation using electroretinogram or behavioral test for visual function [28,29]. In addition, subretinal transplantation of differentiated RPE cells in dystrophic Royal College of Surgeon (RCS) rat instead of sodium iodate-induced degeneration model would provide more solid evidence of the therapeutic effect of cell transplantation. The RCS rat is a well-characterized model of retinal dystrophy in which the primary defect originating in RPE cells leads to blindness as a consequent of photoreceptor degeneration [30].
In conclusion, we could differentiate pigmented cells from hESC under well-defined and reproducible conditions. The putative RPE cells showed characteristic morphologic features of the RPE cells and expressed molecular markers associated with RPE fate. We transplanted these hESC-RPE cells in the subretinal space of rat retinal degeneration model, and the grafted cells were found to survive and integrate within recipient retina up to four weeks after transplantation. Although there are still numerous unanswered questions surrounding stem cell transplantation in retinal degenerative diseases, these results support the feasibility of the hESC-derived RPE cells for cell-based retinal therapies.
Notes
This work was supported by the Mid-career Researcher Program through NRF grant (No. 2009-0084597).
No potential conflict of interest relevant to this article was reported.