 |
 |
- Search
| Clin Exp Reprod Med > Volume 38(2); 2011 > Article |
Abstract
A 58-year-old woman who presented with inguinal hernia for the first time was diagnosed as seminoma and complete androgen insensitivity syndrome (CAIS). The patient received a late diagnosis, and therefore she could not take a proper management. CAIS is a rare X-linked recessive disease with an XY karyotype that is caused by androgen receptor defects. It usually present with primary amenorrhea or inguinal hernia. The risk of malignant transformation of undescended testis increases with age, thus gonadectomy should be performed after puberty. We present a case of large advanced seminoma in a woman with CAIS who was neglected and diagnosed lately.
Complete androgen insensitivity syndrome (CAIS), or testicular feminization, is a rare X-linked recessive disease characterized by variable defects in virilization of individuals with male karyotype (46,XY) and an absence of sex chromatin. CAIS is caused by mutations in the androgen receptor gene, resulting in impaired embryonic sex differentiation and producing a female external phenotype.
Most patients with CAIS are diagnosed at puberty with primary amenorrhea. Although their risk of malignancy is extremely low, malignant transformation of dysgenetic male gonads (undescended testes) has shown a significant association with aging. We describe here a 58-year-old patient, never previously diagnosed with CAIS, who presented with a large, late stage malignant seminoma as the primary presentation of CAIS.
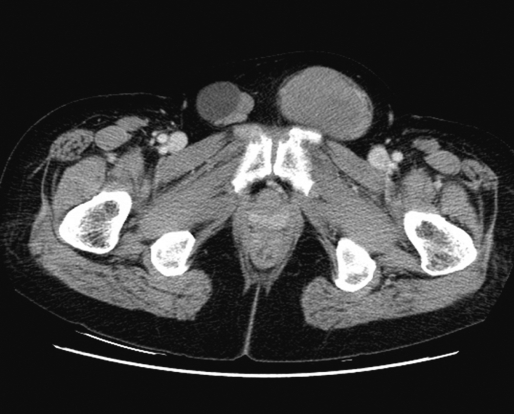
A 58-year-old woman was referred from a primary clinical to the Department of General Surgery at Asan Medical Center (Seoul, Korea) for an inguinal hernia. She had noticed reducible swellings on both inguinal areas for 10 years but did nothing until the swellings became problematic. These swellings had increased 7 months earlier, accompanied by pain. Ultrasound examination showed a well-defined cyst in the right inguinal area, and a mass with cystic and solid portions in the left inguinal area. Computed tomography showed a 7.4 cm round homogeneously enhanced mass in the left inguinal canal with an enlarged lymph node in the left para-aortic area (Figure 1).
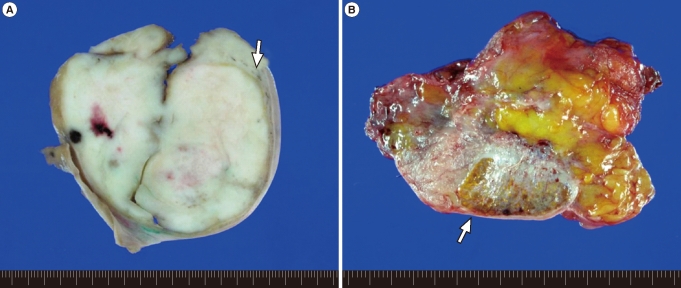
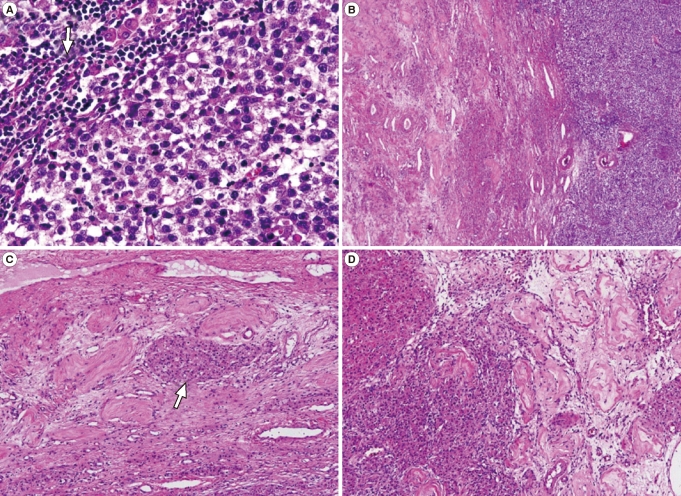
The mass excised from the left inguinal area was well-defined and firm, measuring 7├Ś7├Ś6 cm in size. The cut surface was homogenously creamy white and vaguely lobulated with focal hemorrhage (Figure 2A). The mass in the right inguinal canal was heterogeneously golden yellow to pinkish gray and encapsulated (Figure 2B). Microscopically, the mass of left inguinal area consisted of large uniform cells growing in broad sheet divided by thin septa which infiltrated by lymphocytes. The nuclei of the tumor cells were centrally located and round to polygonal with finely granular chromatin and prominent nucleoli. The cytoplasm was eosinophilic to clear and had distinct cell border (Figure 3A). These features were consistent with seminoma of classic type. Adjacent to the mass was soft tissue composed of atrophic hyalinized seminiferous tubules and interspersed Leydig cell nests in between, compatible with testicular tissue (Figure 3B, 3C). The mass from right inguinal canal also had testicular tissue with atrophic hyalinized seminiferous tubules, but the leydig cell nests were larger and more abundant, consistent with leydig cell hyperplasia (Figure 3D). This mass was removed surgically, as was the reducible mass in the right inguinal canal, followed by herniorrhaphy.
Postoperatively, the surgeon in charge requested a detailed examination of this patient by a gynecologist for further evaluation and treatment. The patient was amenorrheic for her entire life, is unmarried, and a virgin. She was 170 cm in height and weighed 80 kg. Physical examination showed that her breasts were normally developed, but her pubic hair and axillary hair were sparse. She had slightly enlarged external genitalia with a blindly-ending vagina about 2 cm long. Chromosomal analysis showed a 46,XY karyotype, but none of her previous gynecological examinations had diagnosed any distinct features. Her serum testosterone concentration was 0.25 ng/mL (normal male range, 2.6-15.9 ng/mL) and her estradiol concentration was 12.1 pg/mL (normal male range, 0-44 pg/mL). Based on these findings, the patient was diagnosed with AIS. Because computed tomography suggested metastasis along the left para-aortic lymph node, adjuvant radiotherapy and chemotherapy were recommended.
CAIS, originally called complete testicular feminization, is a phenotype resulting from a defect in androgen receptor function and causes peripheral androgen resistance. Since the androgen receptor is encoded by a gene located on chromosome Xq11-12, CAIS is an X-linked recessive disease. It is characterized as a male karyotype with a normal female phenotype, such as female breast development, although there is little or no axillary and pubic hair. The external genitalia appear normal or slightly underdeveloped and the vagina is of varying length, but is usually shortened and with a blind ending. Internal genitalia, including the cervix and uterus, are absent except for undescended testes in the abdominal cavity or inguinal or labia majora along the normal course of testicular descent.
These patients have a plasma testosterone concentration within the normal range in males. However, some patients have elevated plasma testosterone due to increased stimulation by luteinizing hormone. In contrast, our patient had a decreased testosterone level, probably due to her completely atrophied testes.
The prevalence of CAIS in the general population ranges between 1 in 20,000 and 1 in 60,000 in females. It is the third most frequent cause of primary amenorrhea and is the most common form of male pseudohermaphroditism.
Fortunately, CAIS is usually diagnosed at puberty after an individual presents with primary amenorrhea or an inguinal hernia. A few retrospective studies have estimated that 0.8% to 2.4% of girls with inguinal hernias have CAIS [1]. Therefore, phenotypic female infants with an inguinal hernia should be fully evaluated. Lack of awareness of the association between inguinal hernias in girls and CAIS has often led to failure to make an early diagnosis, particularly in the past.
The risk of malignancy in patients with CAIS increases with age. For example, 3.6% of 25-year-old and 33% of 50-year-old AIS patients are likely to have tumors [2]. Tumors in these patients are due to undescended testes, accounting for 10% of patients with testicular tumors [3]. The two most frequent types of testicular tumors associated with AIS are Sertoli cell and germ cell tumors. Several long-term follow-up studies have shown that the timing of gonadectomy in women with CAIS can be delayed until sexual maturation is complete, because patients with CAIS have a normal pubertal growth spurt and feminize at the time of expected puberty, and usually do not develop malignant tumors until puberty [4]. Therefore, prophylactic gonadectomy after puberty is recommended [5]. Routine ultrasound examination is recommended to monitor potential malignant changes in the gonads of these patients.
We describe here an older woman with advanced seminoma who presented with a bilateral inguinal hernia and was first diagnosed with CAIS. The diagnosis of CAIS was delayed in this patient, and she failed to receive appropriate care, resulting in the development of a seminoma and its progression to an advanced stage.
References
1. Sarpel U, Palmer SK, Dolgin SE. The incidence of complete androgen insensitivity in girls with inguinal hernias and assessment of screening by vaginal length measurement. J Pediatr Surg 2005;40:133-136.PMID: 15868573.


2. Manuel M, Katayama PK, Jones HW Jr. The age of occurrence of gonadal tumors in intersex patients with a Y chromosome. Am J Obstet Gynecol 1976;124:293-300.PMID: 1247071.


3. Abratt RP, Reddi VB, Sarembock LA. Testicular cancer and cryptorchidism. Br J Urol 1992;70:656-659.PMID: 1362513.


4. Cheikhelard A, Morel Y, Thibaud E, Lortat-Jacob S, Jaubert F, Polak M, et al. Long-term followup and comparison between genotype and phenotype in 29 cases of complete androgen insensitivity syndrome. J Urol 2008;180:1496-1501.PMID: 18710728.


5. Purves JT, Miles-Thomas J, Migeon C, Gearhart JP. Complete androgen insensitivity: the role of the surgeon. J Urol 2008;180:1716-1719.PMID: 18715581.


Figure┬Ā1
Enhanced computed tomography scan showed round homogeneous mass on left inguinal area and lobulated contouring mass in the opposite site.
Figure┬Ā2
Gross photograph of masses. (A) The mass in the left inguinal area shows homogenous creamy white and lobulated cut surface. The gray soft tissue is present between the mass and capsule (arrow). (B) The excised specimen from the right inguinal canal is golden yellow and pinkish gray mass (arrow).
Figure┬Ā3
Microscopy of masses (H&E stain) (A) The mass of left inguinal area consists of large uniform cells with central round to polygonal nuclei and distinct cell border with eosinophilic to clear cytoplasm. The thin septa dividing the tumor cell sheets are infiltrated by lymphocytes (arrow) (├Ś400). (B) Adjacent to the mass (right) is testicular tissue (left) composed of (├Ś100) (C) atrophic hyalinized seminiferous tubules and interspersed Leydig cell nests (arrow) in between (├Ś200). (D) The mass in right inguinal canal show Leydig cell hyperplasia (left) and atrophic hyalinized seminiferous tubules (right) (├Ś200).
-
METRICS

- Related articles in Clin Exp Reprod Med
-
Clinical characteristics in Taiwanese women with polycystic ovary syndrome2015 September;42(3)





