The effects of blastocyst morphological score and blastocoele re-expansion speed after warming on pregnancy outcomes
Article information
Abstract
Objective
The aim of this study was to investigate associations between the morphology score of blastocysts and blastocoele re-expansion speed after warming with clinical outcomes, which could assist in making correct and cost-effective decisions regarding the appropriate time to vitrify blastocysts and to transfer vitrified-warmed blastocysts.
Methods
A total of 327 vitrified-warmed two-blastocyst transfer cycles in women 38 years old and younger were included in this retrospective study.
Results
The clinical pregnancy rate (CPR) and implantation rate (IR) of transfers of two good-morphology grade 4 blastocysts vitrified on day 5 (64.1% and 46.8%, respectively) were significantly higher than the CPR and IR associated with the transfers of two good-morphology grade 3 blastocysts vitrified on day 5 (46.7% and 32.2%, respectively). No significant differences were found in the CPR and IR among the transfers of two good-morphology grade 4 blastocysts regardless of the day of cryopreservation. Logistic regression analysis showed that blastocoele re-expansion speed after warming was associated with the CPR.
Conclusion
The selection of a good-morphology grade 4 blastocyst to be vitrified could be superior to the choice of a grade 3 blastocyst. Extending the culture of grade 3 blastocysts and freezing grade 4 or higher blastocysts on day 6 could lead to a greater likelihood of pregnancy. Since re-expansion was shown to be a morphological marker of superior blastocyst viability, blastocysts that quickly re-expand after warming should be prioritized for transfer.
Introduction
With refinements in embryo cryopreservation technology in recent years allowing almost all embryos to survive the vitrification/warming process, vitrified-warmed embryo transfer (VET) has become increasingly common. VET is advantageous in that it effectively prevents in vitro fertilization-associated complications such as ovarian hyperstimulation syndrome [1], is associated with better obstetric and perinatal outcomes [2], and maximizes the embryo utilization rate and increases the cumulative pregnancy rate [34], particularly for slowly developing embryos [5] and patients exhibiting early progesterone elevation [6].
Blastulation of human embryos usually occurs on day 5 after fertilization but may be delayed until day 6 or 7. Previous studies have found that the pregnancy rates for fresh transfers performed on day 6 or later were much lower than those performed on day 5 due to declining endometrial receptivity [78]. However, transfers of vitrified day 6 blastocysts have been reported by some investigators to have comparable pregnancy rates to the transfers of vitrified day 5 blastocysts [910]. Other studies found the pregnancy rates to be lower [1112]. A meta-analysis comparing vitrified day 6 and day 5 blastocysts showed lower pregnancy rates and live birth rates in vitrified day 5 blastocysts. However, when the analysis was restricted to studies comparing day 6 and day 5 blastocysts of similar morphology, no difference was found [13]. The authors suggested that blastocyst expansion extent (regardless of the number of days after fertilization) or blastocyst re-expansion capacity after warming may affect the pregnancy outcomes in vitrified-warmed blastocyst transfer cycles.
Gardner and Schoolcraft [14] set up an easy and noninvasive three-parameter scoring system depending on blastocyst expansion, inner cell mass (ICM), and trophectoderm (TE) development. Several reports have demonstrated that TE morphology is significantly related to pregnancy outcomes [151617]. However, Goto et al. [18] reported no distinction between the relative importance of the ICM and the TE within the same blastocyst expansion in VET cycles. Post-warming blastocysts have experienced multiple morphological changes in the process of vitrification/warming. First, the blastocyst is subjected to artificial shrinking for blastocoele dehydration before vitrification, then experiences the action of cryoprotectants during cooling, followed by rehydration via the removal of cryoprotectants during warming. The shrinkage and swelling of the blastocoele may result in cell damage or even death. It is then difficult to evaluate blastocyst quality in comparison to a fresh blastocyst. The extended culture of post-warming blastocysts provides a chance to evaluate blastocoele re-expansion, which may take place within 1 to 2 hours after warming [19]. However, little information has been published on correlations between blastocoele re-expansion speed, the morphological score of blastocysts, and the pregnancy outcomes of vitrified-warmed blastocyst transfers. Furthermore, no uniform criteria for blastocyst cryopreservation exist, although blastocysts with poorer morphological grades did not qualify for cryopreservation in many studies [920].
The objective of this study was to investigate the correlations of blastocyst morphological score and blastocoele re-expansion speed with the clinical outcomes of vitrified-warmed blastocyst transfer cycles. In particular, we compared the clinical outcomes and the percentage of fast blastocoele re-expansion (≥50% blastocoelic cavity formation during 2 hours after warming) in transfers of good-morphology grade 3 blastocysts with those of grade 4 blastocysts vitrified on day 5 after fertilization, in order to obtain information supporting the selection of appropriate blastocysts for vitrification and transfer.
Methods
1. Subjects
This study was a retrospective analysis of vitrified-warmed two-blastocyst transfer cycles from January 2014 to April 2015 in the reproductive center of the 105th Hospital of PLA. The inclusion criteria were: (1) patients ≤38 years of age; (2) non-obese patients with normal levels of basal follicle-stimulating hormone (FSH) (mean, 7.1±3.1 IU/L) and luteinizing hormone (mean, 5.3±2.5 IU/L); (3) blastocysts obtained from the first oocyte retrieval cycle and cryopreserved by vitrification; and (4) patients who underwent the successful transfer of two blastocysts equal or superior to grade 3CC according to Gardner's criteria [14]. A total of 327 VET cycles satisfied the inclusion criteria. All patients provided written informed consent for the procedures. The study was approved by the Ethics Committee of the 105th Hospital of PLA, Hefei, China (2013085).
Blastocysts were evaluated on the basis of the expansion of the blastocoele in addition to the number and cohesiveness of the ICM and TE, according to Gardner's criteria [14]. A good-morphology blastocyst was defined as having a well-expanded blastocoele (grades 3–6) on day 5 or on day 6 (grades 4–6), a well-defined ICM (subgrades A or B) and a single layer of TE cells surrounding the cavity (subgrades A or B). No changes were made to our laboratory protocols during the study period. According to the blastocyst morphological score and the time of blastocyst freezing (with the day of fertilization termed day 0), the transfer cycles were categorized into six groups: BL-1, two good-morphology grade 3 blastocysts frozen on day 5; BL-2, two good-morphology grade 4 blastocysts frozen on day 5; BL-3, one good-morphology grade 3 blastocyst and one good-morphology grade 4 blastocyst, with both frozen on day 5; BL-4, two good-morphology grade 4 blastocysts frozen on day 6; BL-5, one good-morphology blastocyst and one poor-quality blastocyst; BL-6, two poor-morphology blastocysts.
Transfer cycles involving two quickly re-expanding blastocysts (≥50% blastocoele re-expansion during 2 hours after warming) were defined as the re-expansion group 1. Transfers with one quickly and one slowly re-expanding blastocyst (<50% blastocoele re-expansion) were defined as the re-expansion group 2. Those with no quickly re-expanding blastocysts were defined as the re-expansion group 3. Theoretically, blastocysts exhibiting re-expansion or slow re-expansion after warming were considered to have lower development potential. Good-morphology blastocysts could have been prioritized for warming if more than two blastocysts underwent vitrification during the study. Moreover, the study was limited by the fact that the patients in each group were not matched, and that the re-expansion group 3 had a small sample size.
2. Ovarian stimulation and embryo culture
Ovarian stimulation was carried out according to standard gonadotropin-releasing hormone agonist/FSH, antagonist/FSH, or mini-stimulation protocols. Ovulation was triggered by human chorionic gonadotropin when at least three follicles reached 18 mm in diameter, followed by oocyte retrieval approximately 36 to 38 hours after human chorionic gonadotropin administration. Oocytes were fertilized by conventional in vitro fertilization or intracytoplasmic sperm injection, according to the results of the semen analysis. The fertilized oocytes were individually cultured in 20-µL micro-drops of pre-equilibrated G1-plus medium (Vitrolife, Gothenburg, Sweden) under mineral oil until day 3. The embryos were then transferred into 20-µL micro-drops of pre-equilibrated G2-plus (Vitrolife) until day 5 to 7. All embryos were incubated at 37℃ in 6% CO2.
3. Blastocyst vitrification freezing and warming procedures
In this study, only blastocysts equal or superior to grade 3CC were selected for cryopreservation using a commercial vitrification freezing/warming kit (Kitazato Biopharma Co., Shizuoka, Japan) and all freezing/warming steps were carried out according to the manufacturer's instructions. Briefly, the blastocysts were artificially shrunk for dehydration of the blastocoele by the laser method, and one hole of 10 µm on the zona pellucida was made with one or two laser beams at a site far from the ICM using an irradiation time of 3.0 msec (Octax Eyeware, Bruckberg, Germany). After completing the shrinkage of the blastocoele, blastocysts were placed into equilibration solution for 5 minutes at room temperature, then transferred into the vitrification solution for approximately 45 seconds and loaded into the tip of a hemi-straw carrier, with a very small total volume, and were immediately plunged into liquid nitrogen with protection provided by the plastic cover. For the warming procedure, the straw containing the blastocysts was transferred into 1 mL of thawing solution with 1.0 M sucrose (pre-warmed at 37℃) for 1 minute. Thereafter, the blastocysts were transferred sequentially into dilution solutions with 0.5 M sucrose for 3 minutes and washed twice with washing solution for 5 minutes at room temperature. Post-warming blastocyst survival was defined as >50% of the cells remaining intact [19]. Blastocysts were cultured for 1 to 2 hours in order to assess the extent of blastocoele re-expansion, and then were transferred into the uterine cavity.
In our experience, as described in previously published studies [21], high-quality blastocysts started to re-expand within 1 hour after thawing. Blastocysts with a blastocoele re-expansion of more than 50% of the original size within 2 hours were defined as quickly re-expanding blastocysts, while those with a blastocoele re-expansion of less than 50% of the original size were regarded as slowly re-expanding blastocysts, and those not showing any sign of re-expansion were classified as non-expanding blastocysts.
4. Endometrial preparation for VET
VET was carried out in hormone replacement cycles or in natural cycles. Endometrial preparation was performed on the third day of menstruation in hormone replacement cycles, which comprised approximately 95% of the cycles. Estradiol valerate tablets (Progynova, Bayer Schering, Guangzhou, China) were given at a dose of 4 to 8 mg per day, with the dose modified according to endometrial thickness and morphology. When the endometrial thickness reached ≥8 mm, 60 mg of progestin (progesterone injection, Xianju pharmacy Co., Taizhou, China) was administered intramuscularly once a day for 5 days.
5. Embryo transfer and pregnancy diagnosis
Two blastocysts were transferred into the uterine cavity with a Wallace catheter (Wallace, Brisbane, Australia) under abdominal ultrasound guidance. Observation of a gestational sac with a beating fetal heart on day 30 to 35 after embryo transfer was defined as a clinical pregnancy. The number of gestational sacs with a beating fetal heart per number of embryos transferred was defined as the implantation rate (IR). Embryo developmental arrest or spontaneous abortion following less than 12 weeks of gestation was defined as miscarriage.
6. Statistical analysis
Comparisons of continuous variables were carried out using one-way analysis of variance when the data were normally distributed. For non-continuous variables, statistical comparisons were carried out using the chi-square test or Fisher's exact test with multiple-comparison tests where appropriate. The interaction of blastocoele re-expansion speed (re-expansion groups 1–3) with clinical outcomes was examined by logistic regression analysis, allocating numerical scores of 1 to 3 for re-expansion groups 1 to 3. The p-values <0.05 were considered to indicate statistical significance.
Results
No significant differences were found among groups BL-1 through BL-6 regarding female age at embryo freezing, the proportion of primary infertility, infertility duration, the intracytoplasmic sperm injection ratio, the cause of infertility, or endometrial thickness (Table 1).
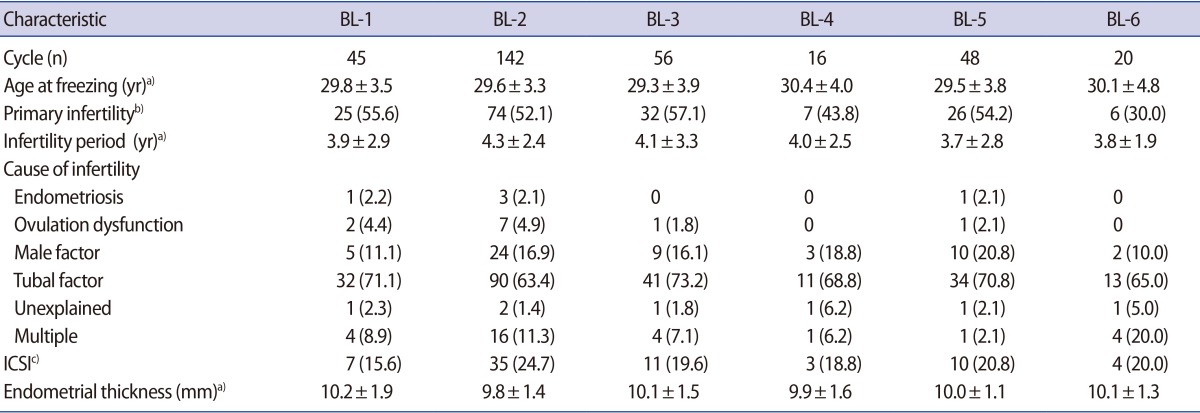
Patients' characteristics of vitrified-warmed blastocysts transfer cycles according to blastocyst quality score (BL-1 to BL-6)
The total clinical pregnancy rate (CPR) of transfers with two good-morphology blastocysts (BL-1, BL-2, BL-3, and BL-4 groups) was 60.6% (157/259), which was significantly higher than that of transfers with two poor-morphology blastocysts (BL-6 group, 35.0%) (p<0.05). The total IR of transfers with two good-morphology blastocysts (BL-1, BL-2, BL-3, and BL-4 groups) was 43.4% (225/518), which significantly higher than when the transfer involved either one good-morphology blastocyst (BL-5 group, 31.3%) (p<0.05) or no good-morphology blastocysts (BL-6 group, 20.0%) (p<0.01).
The CPR and IR of transfers of two good-morphology grade 4 blastocysts frozen on day 5 (BL-2 group: 64.1% and 46.8%, respectively) were significantly higher than the corresponding values for two good-morphology grade 3 blastocysts frozen on day 5 (BL-1 group: 46.7% and 32.2%, respectively), only one good-morphology blastocyst (BL-5 group: 47.9% and 31.3%, respectively) and two poor-morphology blastocysts (BL-6 group: 35.0% and 20.0%, respectively) (p<0.05). Moreover, no significant differences were found in the CPR and IR among the transfers of two good-morphology blastocysts involving grade 4, regardless of the day of cryopreservation (BL-2, BL-3, and BL-4 groups). No significant differences were observed in the rates of miscarriage and multiple pregnancies among the six groups (Table 2).
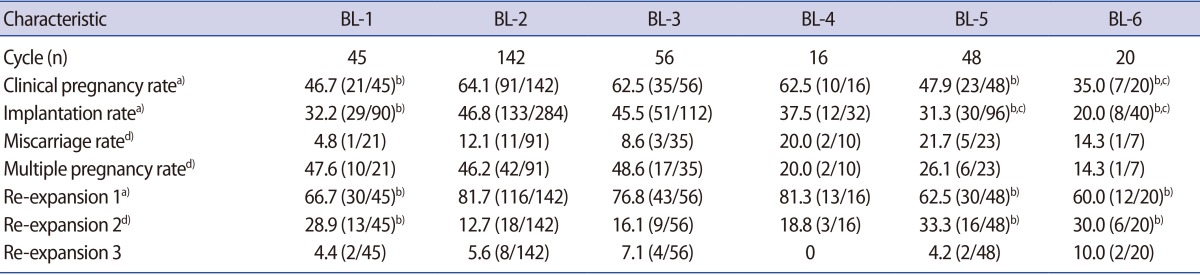
Pregnancy outcomes and blastocoele re-expansion of vitrifed-warmed blastocysts transfer cycles according to blastocyst quality score (BL-1 to BL-6)
Fast re-expansion was exhibited by 84.6% of the blastocysts (553/654) after warming, and 94.5% of the transfer cycles (309/327) contained at least one quickly re-expanding blastocyst. The CPR and IR in re-expansion group 1 (two quickly re-expanding blastocysts) were significantly higher than in re-expansion group 2 (one quickly and one slowly re-expanding blastocyst) and re-expansion group 3 (no quickly re-expanding blastocysts) (CPR: 62.3% vs. 44.6% vs. 33.3%, p<0.05; IR: 44.3% vs. 30.8% vs. 19.4%, p<0.01). Logistic regression analysis also showed that blastocoele re-expansion speed after warming was associated with the CPR (odds ratio, 0.56; 95% confidence interval, 0.35–0.78), with numerical scores of 1 to 3 allocated for re-expansion groups 1 through 3. No statistically significant differences were found in the rates of miscarriage and multiple pregnancies among the three groups (Table 3). In addition, 6.1% (40/654) of vitrified-warmed blastocysts did not re-expand within 2 hours, and one ongoing clinical pregnancy was established from the 10 blastocysts without blastocoele re-expansion that were transferred in re-expansion group 3.
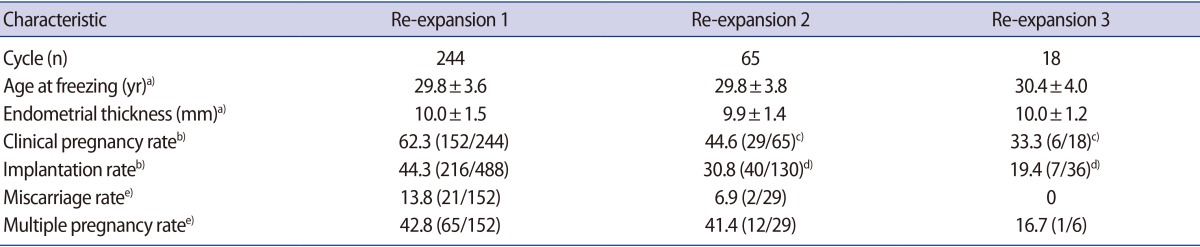
Pregnancy outcomes of vitrified-warmed blastocyst transfer cycles according to blastocoele re-expansion speed after warming
The percentage of cycles involving two quickly re-expanding blastocysts in the BL-2 group (good-morphology grade 4 blastocysts) was higher than in the BL-1 group (good-morphology grade 3 blastocysts) (p<0.05), which was consistent with the clinical pregnancy rates (Table 2, Figure 1).

The relationship of the blastocyst morphological score with the blastocoele re-expansion speed after warming and the CPR. BL-1, two good-morphology grade 3 blastocysts frozen on day 5; BL-2, two good-morphology grade 4 blastocysts frozen on day 5; BL-3, one good-morphology grade 3 blastocyst and one good-morphology grade 4 blastocyst, with both frozen on day 5; BL-4, two good-morphology grade 4 blastocysts frozen on day 6; BL-5, one good-morphology blastocyst and one poor-quality blastocyst; BL-6, two poor-morphology blastocysts; CPR, clinical pregnancy rate; re-expansion 1 (%), the percentage of cycles involving two quickly re-expanding blastocysts.
Discussion
The results of our study showed that transferring two good-morphology grade 4 (4AA/4AB/4BA/4BB) blastocysts frozen on day 5 achieved a higher CPR and IR than was obtained when transferring grade 3 (3AA/3AB/3BA/3BB) blastocysts frozen on day 5 and either one or no good-morphology blastocysts. No significant difference was found in the CPR or IR when two good-morphology grade 4 blastocysts were transferred, regardless of the day of cryopreservation. Logistic regression analysis showed that blastocoele re-expansion speed after warming was associated with the CPR.
Previous studies have evaluated the influence of blastocyst morphology on pregnancy rates. Gardner et al. [22] reported that the blastocyst morphological score had a predictive value for embryo development competence, and a significantly higher CPR was achieved when two top-scoring blastocysts were transferred on day 5, in comparison with the transfers of either one top-scoring blastocyst or no top-scoring blastocysts. Veleva et al. [23] analyzed factors affecting the outcome of VET cycles, showing that transfer of the embryo with the highest quality and viability after warming led to better clinical outcomes. Our study confirmed these findings, showing that when two good-morphology blastocysts were transferred, the CPR and IR were higher than when either one good-morphology blastocyst or no good-morphology blastocysts were transferred.
In a study of 1,488 single frozen-thawed blastocyst transfer cycles, Goto et al. [18] found a significant correlation between blastocyst quality and the CPR. However, neither ICM nor TE affected the pregnancy outcomes within the same grade 3 or grade 4 blastocysts. In the present study, the transfer of two good-quality grade 4 blastocysts frozen on day 5 achieved a higher CPR and IR than was achieved when two good-quality grade 3 blastocysts frozen on day 5 were transferred, suggesting that the blastocyst grade (morphology and blastocyst expansion extent) before freezing affected pregnancy outcomes in vitrified-warmed blastocyst transfer cycles. This result was similar to those reported by Goto et al. [18], who showed that the CPR associated with the transfer of grade 3 (3AA/3AB/3BB) blastocysts was 49.17%, which was lower than that of grades 6, 5, 4AA, 4AB, and 4BA (67.09%) in patients 38 years old and younger. These results suggest that embryo development to the expanded blastocyst stage (higher than grade 4) is an important parameter for pregnancy outcomes.
More cycles in which two quickly re-expanding good-morphology blastocysts were transferred involved grade 4 blastocysts than grade 3 blastocysts, while similar CPRs were observed. Transfer cycles containing at least one quickly re-expanding blastocyst achieved an approximately two-fold higher CPR and IR than those without quickly re-expanding blastocysts, which was consistent with the results of Shu et al. [24]. This suggests that the extended culture of early blastocysts to the expanded blastocyst stage for freezing may enhance their ability to tolerate cryopreservation agents and facilitate blastocyst survival and re-expansion after warming. In addition, good-morphology grade 4 blastocysts had a greater number of TE cells, better compaction of the ICM, and a larger blastocoele volume, resulting in thinning of the zona pellucida, which could facilitate implantation in vitrified-warmed blastocyst transfer cycles in comparison with good-morphology grade 3 blastocysts. Previous studies have revealed that extending the culture of post-warming expanded blastocysts and transferring spontaneously hatching/hatched blastocysts can result in higher implantation and pregnancy rates [5]. Therefore, the selection of expanded grade 4 blastocysts to be frozen on day 5 or extending the culture of post-thaw grade 3 or 4 blastocysts to the hatching/hatched stage for transfer may lead to better pregnancy outcomes.
In addition, Hashimoto et al. [12] reported that IR of vitrified–warmed blastocysts frozen on day 5 (normally developing embryos) was higher than that of those that required culture to day 6, and the incidence of abnormal spindles in the growth-retarded blastocysts was higher than that in normally developing blastocysts. However, in the present study, no significant difference was found in the CPR or IR among the transfers of two good-morphology grade 4 blastocysts regardless of the day of cryopreservation. This difference may have been due to the small sample (16 cycles) of transfers of good-morphology grade 4 blastocysts frozen on day 6.
Blastocysts that did not show significant re-expansion after warming were more susceptible to the procedure of vitrification freezing and warming, and had a lower survival rate than expanded blastocysts [19]. However, it was noteworthy that one ongoing clinical pregnancy was established from 10 transferred blastocysts that did not exhibit blastocoele re-expansion 2 hours post-warm culture, suggesting that unexpanded blastocysts 2 hours post-warm culture still had some likelihood of implanting on the endometrium. These results agree with those reported in other studies, which showed that some shrunken post-warm blastocysts could develop to the hatched stage after thawing for 5 to 6 hours and that the shrunken blastocyst exhibited sufficient implantation ability [25].
In conclusion, the total IR of transfers with two good-morphology blastocysts was significantly higher than that observed with either one good-morphology blastocyst or no good-morphology blastocysts. Transferring two good-morphology grade 4 blastocysts achieved a higher CPR and IR than was obtained using two good-morphology grade 3 blastocysts. Simultaneously, more transfers involved two quickly re-expanding grade 4 blastocysts than involved quickly re-expanding grade 3 blastocysts. No significant difference in the CPR or IR was observed among the transfers of two good-morphology grade 4 blastocysts. The speed of blastocoele re-expansion, as a superior morphological marker of embryo viability, was associated with pregnancy outcomes. Therefore, selection of a good-morphology grade 4 blastocyst to be frozen on day 5 would be superior to choosing a grade 3 blastocyst, and extending the post-warming culture grade 3 blastocysts to the hatching/hatched stage for transfer may achieve better pregnancy outcomes. If no re-expanded blastocyst is observed 2 hours post-warm culture, the culture time should be extended to 5 hours. If no blastocoele formation occurs nonetheless, additional warming or cancellation of the blastocyst transfer is a judicious choice.
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.