Anti-Müllerian hormone as a predictor of polycystic ovary syndrome treated with clomiphene citrate
Article information
Abstract
Objective
This study aimed to determine the threshold of anti-Müllerian hormone (AMH) as predictor of follicular growth failure in polycystic ovary syndrome (PCOS) patients treated with clomiphene citrate (CC).
Methods
Fifty female subjects with PCOS were recruited and divided into two groups based on successful and unsuccessful follicular growth. Related variables such as age, infertility duration, cigarette smoking, use of Moslem hijab, sunlight exposure, fiber intake, body mass index, waist circumference, AMH level, 25-hydroxy vitamin D level, and growth of dominant follicles were obtained, assessed, and statistically analyzed.
Results
The AMH levels of patients with successful follicular growth were significantly lower (p=0.001) than those with unsuccessful follicular growth (6.10±3.52 vs. 10.43±4.78 ng/mL). A higher volume of fiber intake was also observed in the successful follicular growth group compared to unsuccessful follicular growth group (p=0.001). Our study found the probability of successful follicle growth was a function of AMH level and the amount of fiber intake, expressed as Y=–2.35+(–0.312×AMH level)+(0.464×fiber intake) (area under the curve, 0.88; 95% confidence interval, 0.79–0.98; p<0.001).
Conclusion
The optimal threshold of AMH level in predicting the failure of follicle growth in patients with PCOS treated with CC was 8.58 ng/mL.
Introduction
Polycystic ovary syndrome (PCOS) is found in 5% to 6% of women of reproductive age. It affects women's reproductive capability; thus patients have risks of infertility, miscarriage, and complicated pregnancy. A study revealed that the prevalence of oligoovulation or anovulation in patients with PCOS ranged from 65% to 80% [1]. The most common complaint reported by patients with PCOS was infertility due to chronic anovulation.
Ovarian stimulation followed by ovulation induction is an approach to promote fertility in patients with PCOS [2]. Several methods for this approach have been studied, such as body weight reduction, clomiphene citrate (CC) or gonadotropin administration, and diathermic laparoscopy. Currently, the first-line procedure for ovarian stimulation in patients with PCOS and anovulation is CC treatment [3]. This strategy was reported to be effective in 80% of women with type 2 ovulation; about 50% of women experienced pregnancy after six cycles of administration [1]. In women with successful ovulation induction, occurrence of ovulation was about 71% after the first cycle (50 mg dose), 28% after the second cycle (100 mg dose), and 6% after the third cycle (150 mg dose) [4]. The reported failure rate of ovulation induction in several studies ranged from about 15% to 25% [5]. Ovulation induction failure is defined as the ineffectiveness of CC at the dose of 100 mg or more to induce ovulation, commonly stated as “CC resistant” [6].
Ovulation induction failure associated with CC often occurs in patients with obesity, insulin resistance, hyperandrogenism, and amenorrhea. Patients unresponsive to CC therapy are usually discovered after 3 to 6 months of treatment [4]. If the success rate in patients could be predicted at an earlier time point, then there would be additional time for gynecologists to replace CC with alternative therapeutic modalities, such as gonadotropin or laparoscopy [45]. Factors predicting a successful ovulation with CC treatment include body mass index (BMI), hyperandrogenemia, and age in a nomogram [6]. Ovarian volume and menstrual status are additional factors in predicting the therapeutic response of CC [5]. The ratio of fasting blood glucose level to fasting insulin level could be the marker of CC resistance [7].
As an important regulator in the ovary, anti-Müllerian hormone (AMH) is suspected to affect CC resistance [8]. The specific patterns of expression in the ovary indicate that AMH may play a role during follicle development and function [9]. Produced predominantly by granulosa cells of the preantral and small antral follicles, AMH inhibits follicle recruitment as well as follicle growth and selection dependent on follicle-stimulating hormone (FSH), and also attenuates the FSH-dependent increase of aromatase activity during early follicle development [8]. In addition, AMH has an important role in the ovarian follicular microenvironment associated with ovarian folliculogenesis [10], and AMH showed positive correlation with ovarian sensitivity to FSH [8].
The role of AMH in follicle development was confirmed in a study demonstrating that continuous low dose recombinant FSH administration significantly lowered the AMH level in follicles. A decline of AMH level interrupted the inhibition mechanism in the follicles; therefore they could develop as mature follicles [11]. Previous studies reported that AMH could also be useful in the prediction of ovarian response to CC in obese women with anovulation [12]. Additional studies to determine the most useful clinical value of the hormone level were needed. This study was conducted to define the threshold of the AMH level as a predictor of follicular growth failure in patients with PCOS treated with CC.
Methods
1. Study design
This cross-sectional observational study was conducted in the Endocrinology and Gynecology Clinic in Cipto Mangunkusumo Hospital from June 2013 to April 2014. This study included 50 female subjects with PCOS who were infertile, willing to participate in this study, and had not been under either CC or metformin therapy within the previous 3 months. The Rotterdam consensus was used to exclude subjects without PCOS, although we did not check the level of cortisol, thyroid-stimulating hormone (TSH), and insulin in these patients. Diagnosis of PCOS was based on the Rotterdam consensus criteria (two or three of the following criteria: oligoovulation or anovulation, hyperandrogenemia, and ultrasonographic appearance of polycystic ovaries). We predominantly found oligoovulation or anovulation in our study, and the ultrasonographic appearance of polycystic ovaries, whereas hyperandrogenemia was quite rare. We excluded patients with incomplete data. Subjects were recruited using the consecutive sampling method. This study assessed several variables, which consisted of the age of patient, duration of infertility, cigarette smoking exposure, use of Moslem hijab, sun exposure, fiber intake, BMI, waist circumference, AMH level, 25-hydroxy (25-OH) vitamin D level, and follicular growth. BMI was classified based on the World Health Organization criteria for Asians, where BMI >23.0 kg/m2 is overweight [13]. Anamnesis, physical examination, and transvaginal ultrasonography (TVU) were performed on the eligible study subjects. Venous blood samples were taken to measure serum levels of AMH (AMH Gen II ELISA; Beckman Coulter, Fullerton, CA, USA) and 25-OH vitamin D (chemiluminescent immunoassay method, Liaison 25 OH vitamin D total assay; DiaSorin, Stillwater, MN, USA). CC, at the dose of 100 mg daily, was provided to the subjects to be taken for 5 days starting from day 2 of their spontaneous periods or after progestin-withdrawal bleeding. Follicular growth assessment by TVU was performed at day 12 of the menstrual period. Successful and unsuccessful follicular growth was compared according to the subjects' respective AMH levels. These values were entered into a diagnostic test using statistical analysis and the receiver operating characteristic (ROC) curve. Dietary intake assessment using a 24-hour food recall method was taken by a designated trained interviewer to collect data concerning the amount of daily fiber intake. Analysis on fiber intake was made using the NutriSurvey software program (NutriSurvey for Windows, J. Erhardt, University of Indonesia, Jakarta, Indonesia; http://www.nutrisurvey.de).
2. Inclusion and exclusion criteria
The inclusion criteria were women <40 years, who had been previously diagnosed with PCOS based on Rotterdam consensus criteria (two or three criteria: oligoovulation or anovulation, hyperandrogenemia, and sonographic appearance of polycystic ovaries), had been infertile, were willing to participate in this study, and had not consumed any medications (CC or metformin) within 3 months. We excluded patients with incomplete data.
3. Outcome measures
Primary study outcomes were follicle growth and the threshold level of AMH as a predictor of unsuccessful follicular growth. Successful follicular growth was defined as the presence of one (or more) dominant follicle with a diameter of no less than 17 mm on TVU at day 11 or day 12 of the menstrual period. The secondary outcome was to find a linear function to predict the success (or failure) of follicle growth by analyzing several patient factors such as age, duration of infertility, cigarette exposure, use of Moslem hijab, sun exposure, fiber intake, BMI, waist circumference, and serum AMH and 25-OH vitamin D levels.
4. Statistical analysis
Data were analyzed using the SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA). Valid data were transformed into a distribution table to calculate the means, medians, and the distributions. To determine the threshold level of AMH, statistical analysis and the ROC curve were used. The level of significance α=0.05 was used. Multivariate analysis was established using the binary logistic regression test. Bivariate analysis was done between follicular growth and each of the following variables: age, BMI, waist circumference, AMH level and 25-OH vitamin D level, and the amount of fiber intake. Only variables that showed significant correlation (p-value <0.25) with follicular growth were used for further multivariate analysis.
5. Ethics approval
The study protocol was approved by Ethics Committee of Faculty of Medicine University of Indonesia and Cipto Mangunkusumo Hospital, with Ethical Clearance reference number of 287/H2.F1/ETIK/2013. All participants had been informed and provided written consent prior to enrollment.
Results
The 50 female subjects with PCOS enrolled this study were divided into two groups based on the assessment of follicular growth. Twenty-three subjects (46%) had successful follicular growth and 27 subjects (56%) had unsuccessful follicular growth. Subject characteristics were women of reproductive age (mean±standard deviation [SD], 29.66±5.08 years) with BMI 24.81 kg/m2 (range, 22.23–28.71), and had experienced 4 years (range, 1.25–6.00) of infertility. The mean value of the AMH level was around 7.51 ng/mL, fiber intake was around 9.57 g, 25-OH vitamin D was around 9.50 ng/mL, and waist circumference was around 85.14 cm. Some subjects wore Moslem hijab, had <60 minutes sun exposure, and had no cigarette exposure. The details of subject characteristics are shown in Table 1.
1. Association of AMH level and follicular growth
The mean AMH level was significantly lower (p=0.001) in the successful follicular growth group (mean±SD, 6.10±3.52 ng/mL) compared to that observed in the unsuccessful follicular growth group (mean±SD, 10.43±4.78 ng/mL) (Table 2).
The area under the curve (AUC) showed that AMH level had moderate capacity as the predictor of follicle growth failure (Figure 1).

Based on the receiver operating characteristic (ROC) curve, anti-Müllerian hormone (AMH) levels had the highest area under the curve at 0.75 (95% confidence interval of 0.62–0.88).
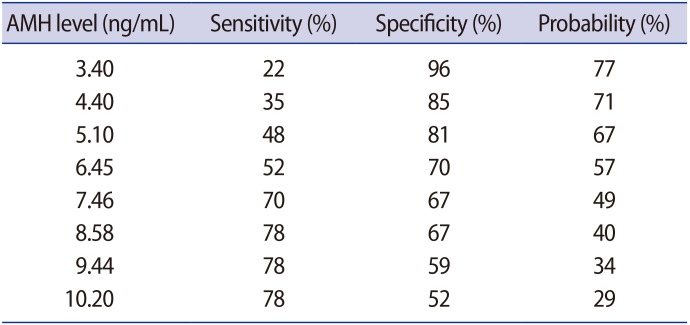
Using the ROC curve, we found the threshold of AMH level for predicting the failure of follicle growth was 8.58 ng/mL, with a sensitivity of 78% and specificity of 67%.
2. Follicular growth as a function of AMH level and fiber intake
An AMH level in the successful follicular growth group was 6.10±3.52 ng/mL and it was found to be significantly lower (p=0.001) compared to that observed in unsuccessful follicular growth group (10.43±4.78ng/mL). The successful follicular growth group showed significant higher fiber intake (11.20±2.68 g) than that observed in unsuccessful follicular growth group (8.47±2.77 g), with p-value, 0.001 (Table 2).
Based on the correlation test, eligible variables for the logistic regression test were BMI, waist circumference, AMH level, fiber intake, use of Moslem hijab, and cigarette exposure. Binary logistic regression test was performed to determine the probability of having successful follicular growth with CC treatment (Table 3), resulting in the following function (Formula 1):
with the probability formulation=
The discriminating capability of the formulation according to the AUC is 0.88 (95% confidence interval [CI] of 0.79–0.98) with p<0.001.
Determination of threshold value was based on the sensitivity and specificity. The threshold of AMH level found in this study was 8.58 ng/mL, and it had a moderately high specificity (67%) and sensitivity (78%), although it only contributed 40% towards predicting failure of follicular growth according to Formula 1 (Table 4).
Discussion
This study was conducted in female subjects with PCOS receiving CC treatment (dose of 100 mg) and observed for successful or unsuccessful follicular growth. The successful follicular growth rate in subjects with CC treatment found in this study was 46% (Table 2), which is consistent with rates (40%–80%) reported in a previous study by Messinis and Milingos [14] .
1. Association of AMH level and follicular growth
The mean level of AMH in subjects with PCOS was 7.51 ng/mL (6.20–8.88) (Table 1). Previous studies in patients with PCOS by Li et al. [15] reported a mean AMH level of 9.85 ng/mL while Catteau-Jonard et al. [11] reported 6.59 ng/mL. Piltonen et al. [16] concluded that the AMH level in patients with PCOS was always two or three times higher than the normal population (16–44 years). In alignment with these reports, meta-analysis by Iliodromiti et al. [17] revealed higher AMH levels in patients with PCOS than in the normal population, thus the AMH level could be used for diagnosing PCOS, with 79% sensitivity and 83% specificity. A study by Sahmay et al. [18] reported a similar result with the AMH level ≥3.9 ng/mL as the cut-off to diagnose PCOS.
The AMH level in the successful follicle growth group was significantly lower (p=0.001) than that of the unsuccessful follicle growth group (Table 2). The AMH level demonstrated a negative correlation with the probability of having a successful follicular growth. This finding concurs with that reported in a previous study by Amer et al. [19], which compared the AMH level in PCOS patients who underwent laparoscopic ovarian diathermy. The AMH level in the ovulation and anovulation groups were significantly different; the level was higher in the anovulation group (p=0.002) [19]. Mahran et al. [20] investigated 187 ovulation induction cycles with CC and revealed higher AMH levels in the anovulation group (p<0.001) than in the ovulation group. di Clemente et al. [21] elaborated the inhibitory mechanism of AMH in the ovulatory process through its effect on specific type II receptors in granulosa and theca cells, in reducing the aromatase enzyme activity and luteinizing hormone receptors in FSH-stimulated granulosa cells, and in interfering with testosterone production by theca cells.
2. Predictor of follicular growth failure
This study aimed to determine the threshold of AMH level as the predictor of follicular growth failure in patients with PCOS treated with CC by using the ROC curve analysis. Based on the result of ROC curve, AMH level had a high AUC and a significant difference between groups (0.75, p=0.001), with 8.58 ng/mL as the threshold with 78% sensitivity and 67% specificity. This finding indicates that PCOS patients with AMH level of <8.58 ng/mL had twofold probability of having a successful follicular growth compared to patients with higher (≥8.58 ng/mL) AMH level.
There were two other, similar studies that attempted to determine the threshold of AMH level. Mahran et al [20]. reported an AUC of 0.809 (p<0.001) for 3.4 ng/mL threshold with 73% sensitivity and 78% specificity, and Amer et al. [19] demonstrated a threshold of 7.7 ng/mL with 78% sensitivity and 76% specificity. These studies had parallel results with ours, corroborating the role of AMH in predicting the failure of follicle growth. The somewhat different thresholds of AMH level among studies were presumably related to the variation of the AMH kits used in those studies.
The AMH level has been known to be a factor related to the follicular fluid [20], as a higher AMH level in the follicles halts follicle development. This was demonstrated in a study using continuous low dose FSH recombinant therapy that resulted in a significantly decreased AMH level in the follicle. It was suggested that a decreased AMH level would lead to the suppression of the inhibitory mechanism in the follicle; thus it could then develop into a dominant follicle [11]. Amer et al. [19] reported that after laparoscopic ovarian diathermy, the AMH level in subjects with ovulation was 25% lower than subjects with no ovulation. This resulted from the break up of the follicles after a diathermic procedure. In addition, the study also found 15% reduction in the AMH level in the group receiving only CC [19]. Highly elevated AMH in follicular fluid from PCOS patients and not age-matched with normal controls is suggesting an intrinsic abnormality in the ovarian follicles themselves in PCOS, which could contribute to disordered folliculogenesis [22].
In comparison with a similarly designed study by Mahran et al. [20], our study differed in terms of CC administration. In our study, a 100 mg dose of CC was administered, while Mahran et al. provided 187 cycles of CC in a total of 60 patients, to whom CC was given gradually until a dose of 150 mg was reached. The rate of successful ovulation was found to increase if CC was given continuously until six cycles or if the doses were increased [214]. Mahran et al. evaluated ovulation when the mid-luteal progesterone was ≥12 ng/mL and by a follicle tracking examination. Our study used only the follicle tracking method, which classified follicle size >18 mm as the indicator of the ovulation process. This study conducted additional analyses of other variables. We found that variables such as age, infertility duration, BMI, waist circumference, 25-OH vitamin D level, and cigarette exposure did not determine the follicular growth. The amount of fiber intake was significantly different between groups (p=0.001), so that the higher the fiber intake, the higher the successful rate of follicular growth.
The association of ovulation with nutrition was previously discussed in a study by Gaskins et al. [23]. That study suggested the correlation of a high fiber intake with an increase of ovulation rate (p<0.001), although the study was not conducted in patients with PCOS [23].
The other variable that has been considered to play a role in the ovulation process in PCOS is 25-OH vitamin D. A recent study demonstrated that 60% to 75% of women with PCOS tended to have a 25-OH vitamin D deficiency (deficiency defined as having <20 ng/mL of 25-OH vitamin D) [24]. This finding was similar to our result showing 94% women with PCOS had 25-OH vitamin D <20 ng/mL, but we did not find any significant difference for vitamin D levels between ovulation and not ovulation group.
Obesity has been known to be a factor affecting ovulation [5]. In a study by Imani et al. [6], the obese and non-obese subjects showed a significant difference in ovulation rate (p<0.001). Another study proposed that weight reduction by diet modification would improve ovulation [25]. In this study there were equivalent results to these previous studies, indicating a BMI difference of 24.74 kg/m2 in the successful follicular growth group and 26.76 kg/m2 in the unsuccessful follicular growth group, although the difference was statistically insignificant (p=0.2).
This study determined a threshold AMH level as a predictor of follicular growth failure following CC treatment. Despite the high sensitivity and specificity of the AMH threshold level (i.e., 8.58 ng/mL), its clinical application would result in only 40% probability of having follicular growth. Consequently, the application of AMH as a predictor of follicular growth failure in patients with PCOS treated with CC seems to be most beneficial in specific populations, like patients with PCOS in Indonesia that are mostly lean, vitamin D deficient, and lacking in signs of hyperandrogenism. Our finding that fiber intake was an influential variable in predicting the successful follicular growth was interesting. However, calculating or estimating the daily amount of fiber intake in clinical settings would be complicated, and fiber intake would be unsuitable for predicting successful follicular growth. Therefore, it was considered as one component in the successful follicular growth probability table according to various AMH levels and as preliminary data for further studies. The patients' symptoms like oligoovulation or anovulation could be related to high cortisol level, or also high production of TSH and insulin, even though the clinical signs did not include hypercortisolism, high TSH levels, or glucose in-tolerance. We did not check the level of cortisol, TSH, and insulin, which is a weakness of this study. Further studies are needed to assess those variables in a normal population as a comparison to our study, to validate the predictors found in this study, and to observe thoroughly the effects of fiber intake on the follicular growth in patients with PCOS with a larger prospective cohort or randomized controlled trial.
In conclusion, this study found that AMH level was the potential predictor of follicular growth failure in patients with PCOS treated with CC. The threshold AMH level was 8.58 ng/mL with 78% sensitivity and 67% specificity (AUC, 0.75; 95% CI, 0.62–0.88). Age, infertility duration, BMI, waist circumference, 25-OH vitamin D level, and cigarette exposure were not different between the two groups. There was a significant difference in fiber intake between the successful and unsuccessful follicular growth groups, which was 11.20±2.68 g and 8.47±2.77 g, respectively.
Acknowledgments
The authors of this research paper gratefully acknowledge Natasya Prameswari, Irene Sinta Febriana, Prasetio Adinugroho, Amalia Shadrina, and Mrs. Liana W. Sutanto for their assistance and advice in preparing this manuscript.
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.