Efficient isolation of sperm with high DNA integrity and stable chromatin packaging by a combination of density-gradient centrifugation and magnetic-activated cell sorting
Article information
Abstract
Objective
This study was carried out to investigate the correlations of the sperm DNA fragmentation index (DFI) with semen parameters and apoptosis, and to investigate the effects of density-gradient centrifugation (DGC) and magnetic-activated cell sorting (MACS) on reducing the proportion of sperm with DNA fragmentation and protamine deficiency.
Methods
Semen analysis and a sperm DNA fragmentation assay were performed to assess the correlations between semen parameters and the DFI in 458 semen samples. Sperm with progressive motility or non-apoptosis were isolated by DGC or MACS, respectively, in 29 normozoospermic semen samples. The effects of DGC or MACS alone and of DGC and MACS combined on reducing the amount of sperm in the sample with DNA fragmentation and protamine deficiency were investigated.
Results
The sperm DFI showed a significant correlation (r=–0.347, p<0.001) with sperm motility and morphology (r=–0.114, p<0.05) but not with other semen parameters. The DFI (11.5%±2.0%) of semen samples was significantly reduced by DGC (8.1%±4.1%) or MACS alone (7.4%±3.9%) (p<0.05). The DFI was significantly further reduced by a combination of DGC and MACS (4.1%±1.3%, p<0.05). Moreover, the combination of DGC and MACS (1.6%±1.1%, p<0.05) significantly reduced the protamine deficiency rate of semen samples compared to DGC (4.4%±3.2%) or MACS alone (3.4%±2.2%).
Conclusion
The combination of DGC and MACS may be an effective method to isolate high-quality sperm with progressive motility, non-apoptosis, high DNA integrity, and low protamine deficiency in clinical use.
Introduction
It has been reported that DNA fragmentation may be caused by an exposure to high levels of seminal reactive oxygen species (ROS), failure of the antioxidant defense system [12], or apoptosis [134]. Sperm DNA damage by ROS is associated with chromatin packaging deficiencies and apoptosis. Protamine is an important molecule involved in packaging sperm DNA to protect sperm DNA from sources of external damage, such as ROS. Therefore, the percentage of sperm with a protamine deficiency has been found to show a positive correlation with the sperm DNA fragmentation rate [5]. Additionally, high ROS levels cause a decrease in the mitochondrial membrane potential, which is an initiating event in the apoptosis cascade [6].
Sperm DNA fragmentation is recognized as a major paternal factor that contributes to the failure of assisted reproductive technology (ART) programs [7], low fertilization rates [89], embryo cleavage [2], implantation [10], and pregnancy rates [1112]. Although a spermatozoon with impaired DNA and normal morphology can fertilize an oocyte, the fertilized oocyte does not successfully proceed through further embryonic development [13], which indicates that the intact paternal genome is required for subsequent development of the embryo [14]. This conclusion is supported by the finding that the percentage of fragmented DNA was positively associated with recurrent pregnancy loss [15] and the miscarriage rate [16].
Several studies have found that the sperm DNA fragmentation index (DFI) had a significant correlation with sperm concentration [517], morphology [51819], and motility [192021]. However, controversy remains regarding the correlations between the DFI and semen parameters because some authors did not observe significant correlations between the sperm DFI and morphology [22], concentration [1821], or motility [18].
It is generally accepted that the cut-off value of the sperm DFI should be ≥30%, because this cut-off value has been reported to be positively correlated with lower rates of pregnancy and delivery [1723]. To select sperm with normal DNA integrity (DFI <30%), intracytoplasmic morphologically selected sperm injection [24], hyaluronic acid-bound sperm selection [2526], and the electrophoresis separation technique [27] are currently under study. Recently, new methods to isolate sperm with high DNA integrity using density-gradient centrifugation (DGC) [2829] and magnetic-activated cell sorting (MACS) have been introduced [30]. DGC significantly reduced the sperm DFI in the semen of intracytoplasmic sperm injection patients [31] and in teratozoospermic patients [32]. MACS not only efficiently reduces the sperm DFI [33], but also effectively separates apoptotic from non-apoptotic spermatozoa [34]. Moreover, a combination of DGC with conventional swim-up [35] or with MACS [34] may be a more effective way to isolate high-quality sperm with better motility and morphology, as well as a lower DFI. Tavalaee et al. [36] suggested that MACS before DGC is more useful for clinical sperm selection than MACS after DGC. However, MACS has limitations regarding sperm concentration and volume for loading due to the small size and volume of the column. Therefore, loading raw semen into the MACS column may reduce the filtering function of MACS and impede its ability to isolate motile non-apoptotic sperm, because dead/apoptotic sperm bind to the MACS column in competition with motile/non-apoptotic sperm.
The objective of this study was to investigate the correlations between the sperm DFI and semen parameters and to establish an efficient method to isolate sperm with high DNA integrity and a low protamine deficiency rate using DGC and MACS.
Methods
This was a prospective study approved by the Institutional Review Board (IRB) of Mamapapa & Baby Clinic (Mamapapa IRB 2014-02). This study was carried out from December 2014 to November 2015.
1. Semen samples and semen analysis
Semen analysis and a sperm DNA fragmentation assay were carried out in 458 semen samples with the patients' approval. Semen samples were collected by masturbation after 3 to 7 days of sexual abstinence. After liquefaction of the semen at room temperature, semen analysis was performed according to World Health Organization (WHO) guidelines (2010) with computer-assisted semen analysis.
2. Sperm DNA fragmentation assay
Sperm DNA fragmentation was evaluated by the sperm chromatin dispersion test using the Halosperm kit (Halotech DNA, Madrid, Spain). Briefly, semen samples were diluted with phosphate-buffered saline to a concentration of 5-10×106/mL, and 20 µL was added to melted agarose and evenly mixed. Then a 20-µL aliquot of the cell/agarose suspension was placed on a pre-coated agarose slide and covered with a glass coverslip (22×22 mm). The slides were allowed to solidify for 5 minutes at 4℃, and then the coverslip was gently removed and the slide was immersed horizontally in acid denaturant for 7 minutes at room temperature. Subsequently, the slide was incubated in lysis buffer at room temperature for 25 minutes. After 5 minutes of washing in distilled water, the slide was dehydrated in a graded ethanol series (70%, 90%, and 100%) for 2 minutes each and subsequently air-dried. The dehydrated slide was stained with the Wright-Giemsa stain and observed under a bright field microscope for halos. Four different dispersion patterns based on halo size were observed: (1) sperm nuclei with a large halo, (2) sperm nuclei with a medium halo, (3) sperm nuclei with a very small halo, and (4) sperm nuclei without a halo. Sperm with a large or medium halo were considered to be normal or non-fragmented, and sperm with a small halo or no halo were considered to have significant DNA fragmentation. The DFI was calculated as the percentage of fragmented sperm cells in a semen sample based on the assessment of at least 200 sperm cells per slide.
3. Density-gradient centrifugation
Of the 458 semen samples, 29 normozoospermic semen samples were selected and each sample was divided into two aliquots. One aliquot was washed with Ham's F-10 medium (Gibco, Life Technologies, Grand Island, NY, USA) and another aliquot was treated with DGC using PureSperm (Nidacon, Gothenburg, Sweden). This discontinuous density gradient consisted of two (80% and 40%) 1-mL layers of PureSperm, and 2 mL of semen was deposited on the 40% layer. The gradient was then centrifuged at 400 g for 15 minutes. After centrifugation, the seminal plasma supernatant was discarded, and the sperm pellet was washed with Ham's F-10 medium by centrifugation for an additional 5 minutes and resuspended.
4. Magnetic-activated cell sorting
Externalization of phosphatidylserine (PS) in the inner plasma membrane is one of the earliest signs of apoptosis [37]. The MACS ART annexin V reagent kit (Miltenyi Biotec, Auburn, CA, USA) was used to filter apoptotic sperm using a binding protein with high affinity for PS, annexin V, conjugated to microbeads [38]. Each washed sample that underwent DGC was subsequently divided into 2 aliquots. One aliquot was kept untouched as the pre-MACS control, while another aliquot was subjected to the MACS technique for the depletion of apoptotic spermatozoa. To perform MACS, briefly, we adjusted the concentration of sperm to 10-50×106/mL with the MACS ART binding buffer. The sperm suspension was centrifuged at 300 g for 4 minutes, and then the pellet was resuspended with 100 µL of the MACS ART Annexin V-conjugated microbead reagent. MACS binding buffer was added, making a final volume of 500 µL, and then mixed well and incubated for 15 minutes at room temperature with agitation. After incubation, the sperm suspension was loaded on a separation column, and the column was placed in a MACS multistand (Miltenyi Biotec) which had been previously rinsed with 1 mL of binding buffer. The sperm fraction retained in the column served as the annexin V-positive fraction (apoptotic sperm), due to binding to annexin V-conjugated microbeads, while the spermatozoa that passed through the column were annexin V-negative spermatozoa with an intact plasma membrane (non-apoptotic sperm).
5. Sperm protamine deficiency assay
Protamines are sperm-specific nuclear proteins that are essential for the packaging of the condensed paternal genome in spermatozoa. Protamine deficiency is likely to be one of the contributing factors to DNA damage. The aniline blue assay is a representative test to assess sperm for protamine deficiency. Briefly, semen smears were prepared by spreading a drop of washed semen onto the glass slide and allowing it to air-dry. All smears were fixed in 3% buffered glutaraldehyde for 30 minutes, stained with 5% aqueous aniline blue, and mixed with 4% acetic acid for 5 minutes. Spermatozoa were observed under oil immersion with bright-field microscopy at 1,000× magnification. The following staining patterns were observed: dark blue or partial blue staining of the sperm head (abnormal, protamine deficiency) and no staining (normal). The protamine deficiency rate was calculated as the percentage of the abnormal sperm cells in a semen sample based on the assessment of 200 sperm cells per slide.
6. Statistics
Results were expressed as mean±standard deviation. The Student's t-test was used to compare the DFI between semen samples with normal and abnormal semen parameters. The Pearson correlation coefficient was used to assess the relationships between the sperm DFI and semen parameters. The sperm DFI and protamine deficiency rates of semen samples treated with DGC or MACS alone and DGC with MACS were compared using analysis of variance, and the rates before and after treatment were evaluated using the paired t-test. Statistical analyses of the DFI and the protamine deficiency rate were performed after arcsine (square root) transformation. The p-values <0.05 were considered to indicate statistical significance. Statistical analysis was performed using SPSS ver. 11.0 (SPSS Inc., Chicago, IL, USA).
Results
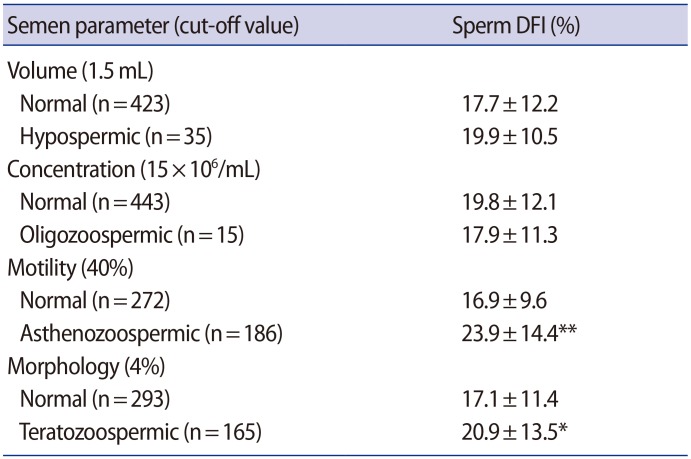
We carried out semen analysis and a Halosperm assay in 458 semen samples to investigate the association of the sperm DFI with normal and abnormal semen parameters (Table 1). No differences were found in the DFI between the semen samples with normal or abnormal concentration and volume. However, the DFI of the semen samples with normal motility (16.9%±9.6%) was significantly lower than that of the asthenozoospermic semen samples (23.9%±14.4%, p<0.001). In addition, the DFI of teratozoospermic semen samples (20.9%±13.5%, p<0.05) was significantly higher than that of the semen samples with normal morphology (17.1%±11.4%).
The correlation coefficients between the sperm DFI and the semen parameters of whole semen samples were assessed (Table 2). Although sperm DFI showed a significant inverse correlations with sperm motility (r=-0.347, p<0.001) or morphology (r=-0.114, p<0.05), no significant correlation was found between the DFI and concentration (r=0.112) and volume (r=0.089).
The experimental process of the present study was illustrated as a diagram (Figure 1). Semen analysis and a sperm DNA fragmentation assay were carried out in 458 semen samples. Of the 458 semen samples, 29 normozoospermic semen samples were selected and each sample was divided into 2 aliquots. One aliquot was washed with medium and another aliquot was treated with DGC. The semen aliquots were subjected to the MACS for the separation into non-apoptotic and apoptotic sperm, and then sperm DFI and protamine deficiency were assessed.

Diagram of experimental procedure. CASA, computer-assisted semen analysis; MACS, magnetic-activated cell sorting.
The DFIs in non-apoptotic and apoptotic sperm in washed semen samples and those that underwent DGC were compared (Figure 2). The DFI of non-apoptotic sperm was significantly lower than the DFI of apoptotic sperm in both washed (7.4%±3.9% vs. 17.2%±3.7%, p<0.05) and DGC semen samples (4.1%±1.3% vs. 20.2%±4.0%, p<0.05). The DFI of washed sperm (11.5%±2.0%) was significantly reduced after treatment with DGC (8.1%±4.1%, p<0.05). The DFIs in the semen samples treated with MACS alone (7.4%±3.9%) or DGC alone (8.1%±4.1%) were significantly greater than the DFI in the semen samples treated with a combination of DGC and MACS (4.1%±1.3%, p<0.05).
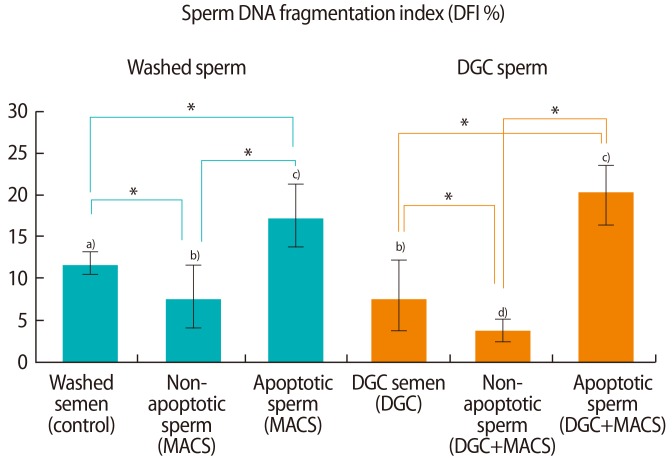
DNA fragmentation index of non-apoptotic and apoptotic sperm in washed and density gradient centrifuged (DGC) groups in 29 normozoospermic semen samples. MACS, magnetic-activated cell sorting. a-d)Results with different superscript alphabets are significantly different (*p<0.05).
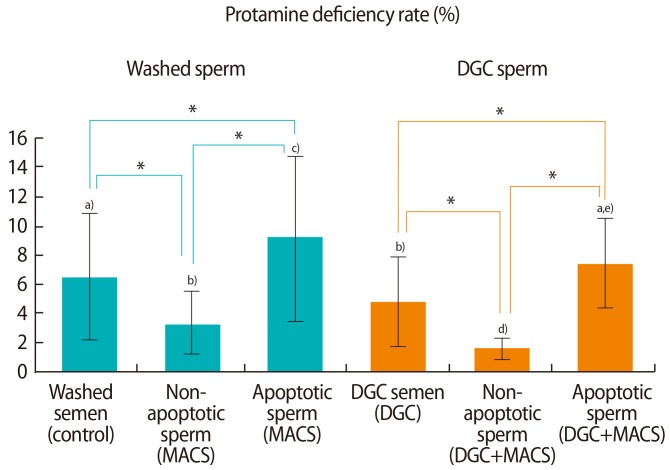
Protamine deficiency rates between non-apoptotic and apoptotic sperm in washed semen samples and those that underwent DGC were compared (Figure 3). The protamine deficiency rates of non-apoptotic sperm in washed and DGC semen samples were 3.4%± 2.2% and 1.6%±1.1%, respectively, which were significantly lower than the rates in apoptotic sperm (9.4%±5.6% and 7.2%±3.7%, respectively, p<0.05). The protamine deficiency rate in washed sperm (6.4%±4.2%) was significantly reduced after treatment with DGC (4.4%±3.2%, p<0.05). The protamine deficiency rates of the sperm samples treated with MACS alone (3.4%±2.2%) or DGC alone (4.4%±3.2%) were significantly higher than the rates of the semen samples treated with a combination of DGC and MACS (1.6%±1.1%, p<0.05).
Discussion
In previous studies, Trisini et al. [17] and Manochantr et al. [5] observed negative correlations between the sperm DFI and sperm concentration (r=−0.16 and r=−0.31, respectively, p<0.05) and motility (r=−0.17 and r=−0.46, respectively; p<0.05). Although we also observed a negative correlation between the DFI and motility (r=−0.347, p<0.05), we did not observe a significant correlation between sperm concentration and the DFI (r=0.112). The discrepancy in the results between the previous studies and the present study may be due to the difference in sexual abstinence period. Trisini et al. [17] collected semen after 2 days of abstinence, and Manochantr et al. [5] collected after 3 days of abstinence, while we collected after a comparatively long abstinence period (3-7 days). If the sexual abstinence period is prolonged, the sperm concentration is increased, while sperm motility and DFI will be decreased due to the increase in dead sperm.
Discrepancies exist in the correlation between the DFI and sperm morphology. In the present study, a significant correlation between sperm DFI and morphology was found, and some authors also have observed the correlation [5181921], while Boushaba and Belaaloui [22] have not. This difference might be caused by differences in the cut-off values for morphology. In the present study, we used the cut-off value of 4% according to the fifth edition (2010) of the WHO guidelines, but Brahem et al. [18] used the cut-off value of 15% according to the fourth edition of the WHO guidelines (1999). Manochantr et al. [5] used a cut-off of 30%, Sivanarayana et al. [19] used a cut-off of 14%, and Boushaba and Belaaloui [22] used a cut-off of 30%. Therefore, further study is needed to clarify how the choice of a cut-off value may affect the correlation between sperm morphology and the DFI.
We observed that sperm motility showed a significantly higher correlation with the DFI than other semen parameters. Similarly, a significant inverse correlation between sperm motility and DFI has been reported in many previous studies [51719202122]. Therefore, no real debate exists regarding the finding that sperm motility has a significant correlation with the DFI. However, Brahem et al. [18] did not observe a significant correlation between the DFI and sperm motility (r = 0.051); nevertheless, they observed a significant decrease in the DFI (approximately 17.0%, p<0.01) following DGC when compared with raw semen (32.8%). In general, DGC is effective in isolating motile sperm from raw semen, resulting in a sharp increase of sperm motility [39]. Therefore, the results of Brahem et al. [18] suggest a close indirect association between the DFI and motility, although they did not observe a significant direct correlation between the DFI and motility. We also observed that the DFI of semen samples was significantly reduced by treatment with DGC (Figure 2). Morrell et al. [40] also reported that the sperm DFI (20.9%±8.1%) was significantly reduced by DGC (12.8%±8.1%, p<0.05). Therefore, the positive effect of DGC on reducing the DFI may be a reasonable result of the increased motility of the sperm sample due to the selection of motile sperm by DGC.
The DFI of the pre-MACS samples was significantly higher than that of the post-MACS samples (Figure 2). This result is similar to that of Degheidy et al. [41], who found that the sperm DFI was significantly reduced in post-MACS samples (9.61%±5.62%) compared with pre-MACS controls (12.43%±6.29%, p<0.05). We also observed that the DFI of non-apoptotic sperm (7.4%±3.9%, p<0.05) isolated with MACS was significantly lower than that of apoptotic sperm (17.2% ±3.7%). This result is supported by previous reports that apoptosis is one of the main causes of sperm DNA fragmentation [134]. Although Degheidy et al. [41] reported that post-MACS samples showed no difference in sperm motility (80.6%±6.9%) compared with control samples (80.9%±7.7%), de Vantery Arrighi et al. [42] reported that the progressive sperm motility of post-MACS semen samples (67.9%±3.8%, p<0.05) was significantly better than pre-MACS control samples (48.4%±2.4%). Additionally, other studies have reported a significant inverse correlation between apoptosis and progressive motility [434445]. Therefore, the positive effect of MACS on the isolation of sperm with high DNA integrity may result from the selection of non-apoptotic sperm with progressive motility. Moreover, we observed that the DFIs of the semen samples treated with MACS alone (7.4%±3.9%) or DGC alone (8.1%±4.1%) were significantly higher than the DFI of the sperm samples treated with the combination of DGC and MACS (4.1%±1.3%, p<0.05). This synergistic effect of DGC and MACS has also been reported in previous studies [3436]. Tavalaee et al. [36] reported that MACS before DGC was more useful for clinical sperm selection than MACS after DGC. Unlike their report, we suggest that MACS after DGC may be more effective, because the MACS column has a small volume (0.5 mL) and limitations in the sperm concentration (10–50×106/mL) for loading. In some oligozoospermic or asthenozoospermic samples, loading of raw semen in the MACS column may result in the collection of insufficient sperm for subsequent DGC treatment. Moreover, the subsequent DGC treatment may result in an additional loss of sperm. We treated the raw semen with DGC in order to collect sufficient motile sperm and exclude immotile sperm and cell debris, and then subsequently treated the samples with MACS, which resulted in the collection of sufficient non-apoptotic sperm with progressive motility and high DNA integrity.
The protamine deficiency rates of non-apoptotic sperm were significantly lower than the rates of apoptotic sperm in both washed and DGC sperm samples (Figure 3), which suggests that a relationship exists between protamine deficiency and apoptosis. The protamine deficiency rates of the sperm samples treated with MACS alone (3.4%±2.2%) or DGC alone (4.4%±3.2%) were significantly reduced when the sperm samples were treated with the combination of DGC and MACS (1.6%±1.1%, p<0.05). This association showed a similar pattern to that of the sperm DFI with DGC and MACS, which suggests that protamine deficiency may be correlated with the DFI. This result is consistent with that of a previous study that reported a positive correlation (r=0.68, p<0.01) between the percentages of protamine deficiency and sperm DNA fragmentation [5], which reflects the fact that protamine is involved in packaging sperm DNA to protect sperm DNA from sources of external damage. Therefore, our results confirm that protamine deficiency is a factor contributing to DNA damage.
In conclusion, treating sperm with a combination of DGC and MACS may be a useful method for isolating non-apoptotic sperm with motility, high DNA integrity, and stable chromatin packaging for clinical use.
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.