Clinical characteristics in Taiwanese women with polycystic ovary syndrome
Article information
Abstract
Polycystic ovary syndrome (PCOS) is one of the most common hormonal endocrine disorders in women of reproductive age. It consists of a heterogeneous collection of signs and symptoms that together form a disorder spectrum. The diagnosis of PCOS is principally based on clinical and physical findings. The extent of metabolic abnormalities in women with PCOS varies with phenotype, body weight, age, and ethnicity. For general population, the prevalence of hyperandrogenism and oligomenorrhea decreases with age, while complications such as insulin resistance and other metabolic disturbances increase with age. Obese women with PCOS have a higher risk of developing oligomenorrhea, amenorrhea, hyperandrogenemia, insulin resistance, and lower luteinizing hormone (LH) to follicle stimulation hormone (FSH) ratios than non-obese women with PCOS. The LH to FSH ratio is a valuable diagnostic tool in evaluating Taiwanese women with PCOS, especially in the diagnosis of oligomenorrhea. Overweight/obesity is the major determinant of cardiovascular and metabolic disturbances in women of reproductive age.
Introduction
Polycystic ovary syndrome (PCOS) is a heterogeneous collection of signs and symptoms that, gathered together, form a disorder spectrum. The diagnosis of PCOS is principally based on clinical and physical findings. Diagnostic criteria and PCOS definitions used by clinicians and researchers are almost as heterogeneous as the disorder itself, and various clinical and biochemical characteristics of PCOS have been reported. Clinical presentations and complications of women with PCOS vary by different phenotypes, age, ethnicity, or body weight. To understand the complications and metabolic disturbances in women with PCOS, several issues should be explored.
Diagnostic criteria for PCOS
A diagnosis of PCOS has lifelong implications for health and wellbeing. Identifying women at risk for PCOS and starting treatment early can effectively prevent some of its long-term complications. Hyperandrogenemia (HA), oligomenorrhea or amenorrhea or anovulation (Oligo-An), and polycystic ovary morphology (PCOM) are the three main criteria currently used to diagnose PCOS. The first comprehensive definition of PCOS arose from a conference sponsored by the National Institute of Health-National Institute for Child and Human Development (NIH-NICHD) in April 1990 [1]. The recommended NIH-NICHD diagnostic criteria were clinical or biochemical evidence of hyperandrogenism and chronic anovulation. However, most gynecologists consider that echographic evidence of PCOM should be an element in PCOS diagnosis. In 2003, new guidelines for diagnosing PCOS were suggested at a joint meeting of the European Society for Human Reproduction and the American Society of Reproductive Medicine. Redefining PCOS to incorporate an appropriate definition of a polycystic ovary was then required [2]. A diagnosis of PCOS can now be reached when at least two findings among HA, Oligo-An, and PCOM are present and after the exclusion of any androgen excess disorders [3].
The Rotterdam 2003 criteria have expanded rather than replaced the 1990 NIH criteria, having added the following two PCOM phenotypes: PCOM with HA and without Oligo-An, and PCOM with Oligo-An and without HA [4]. In our previous study, 18% of Taiwanese women with PCOS had PCOS without HA, and 21% had PCOS without Oligo-An [5]. However, the patient populations with the newly extended phenotypes had less severe ovulatory dysfunction and less androgen excess than patients diagnosed using the 1990 NIH criteria [5]. The prevalence of PCOM has been suggested to be higher than 20% in both Asian and Western women [67]. We found that PCOM was the most common PCOS component in Taiwanese patients; 91% of patients diagnosed with Rotterdam criteria and 85% diagnosed with NIH criteria had PCOM [5]. Regarding clinical presentation, excess androgen has been suggested to have a principal role in diagnostic criteria. Analytical results have demonstrated that PCOS without excess androgen comprises one of the least severe phenotypes [58]. Chae et al. [9] study of Korean women found that PCOS without HA is a common phenotype in Korea; these women are less likely to have metabolic dysfunction, insulin resistance, or elevated blood pressure. Most previous reports support the proposal that excess androgen is the condition directly responsible for the signs and symptoms that are recognized as PCOS [589]. More recently, clinical data have demonstrated that hyperandrogenism is the key component of PCOS, and the Androgen Excess and PCOS (AE-PCOS) Society concluded that PCOS should be first considered as a disorder of androgen excess or hyperandrogenism [4]. The AE-PCOS Society task force recommended that PCOS should be defined by the presence of hyperandrogenism (clinical and/or biochemical), ovarian dysfunction (oligo-anovulation and/or polycystic ovaries), and the exclusion of related disorders (Figure 1) [8].
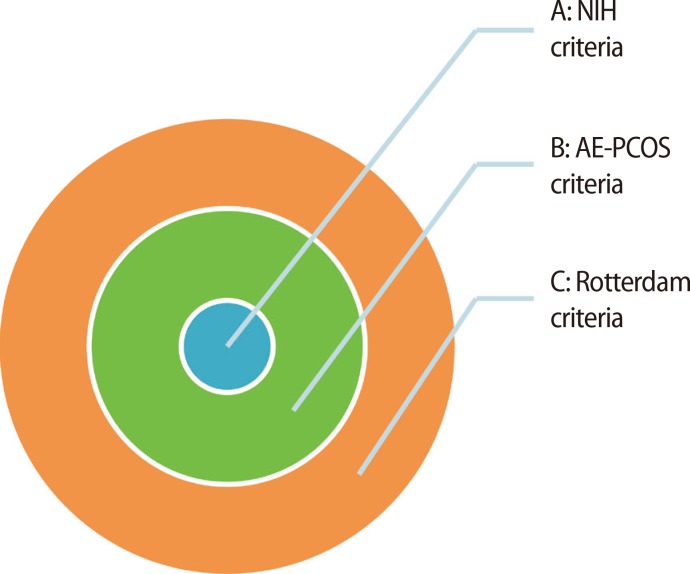
The relationship between Rotterdam 2003 criteria, Androgen Excess and polycystic ovary syndrome (AE-PCOS) criteria, and National Institute of Health (NIH) criteria. NIH criteria, hyperandrogenemia (HA)+oligomenorrhea or amenorrhea or anovulation (Oligo-An)+polycystic ovary morphology (PCOM) and HA+Oligo-An; AE-PCOS criteria, NIH subgroup and HA+PCOM type; Rotterdam 2003 criteria, AE-PCOS subgroup and Oligo-An+PCOM type.
Clinical presentation of hyperandrogenism in Taiwanese women with PCOS
Hyperandrogenism is the major characteristic of women with PCOS. However, the clinical picture of hyperandrogenism might vary with ethnicity. The most apparent signs of androgen excess are external manifestations, including oily skin, acne, hirsutism, android obesity, and androgenic alopecia. Hirsutism is not only a function of circulating androgen levels; it can also be determined by genetic factors. Only 28% to 35% of Asian women with PCOS present with hirsutism [510]. DeUgarte et al. [11] applied the 1990 NIH diagnostic criteria to investigate 271 PCOS patients with average modified Ferriman-Gallwey score (mF-G) scores of 8.0 and determined that 77% had hirsutism. We utilized the same criteria and a similar scoring system to evaluate 95 Taiwanese women, and determined that 35% had hirsutism and average mF-G scores of 4.4. Although 53% of PCOS patients diagnosed under the Rotterdam 2003 criteria had excess clinical androgen, the incidence of hirsutism (28%) was less than that for acne (35%) in our previous study of Taiwanese women [5]. We conducted a study of 627 women in the outpatient clinic of Wan-Fang Hospital. Of these, 55% (346/627) had PCOS, 33% (210/627) had Hyperandrogenemia, 38% (240/627) had acne, 19% (121/627) had hirsutism, and 34% (213/627) had obesity [12]. We found that obese women, despite having higher serum testosterone levels, had a lower incidence of acne than non-obese women; however, this relationship was not found for hirsutism [12]. Clinical presentation of hyperandrogenism might relate to ethnic difference, given that the prevalence of hirsutism in Taiwanese women with PCOS is lower, but the prevalence of acne is higher than Caucasian women with PCOS [5].
Insulin resistance and glucose intolerance in Asian women with PCOS
Insulin resistance and glucose intolerance exert a major long-term health impact on women with PCOS. Women with PCOS have a 5-fold increase in the risk of developing metabolic syndrome (MetS) compared with women without PCOS even after controlling for age and body mass index (BMI), suggesting PCOS alone is an independent risk factor for MetS [13]. Legro et al. [14] studied 254 women with PCOS and found the impaired glucose tolerance (IGT) prevalence was 31.1% and the type 2 diabetes prevalence was 7.5%. Shi et al. [15] reported in 1,040 Chinese women with PCOS an IGT prevalence of 17.2% and a type 2 diabetes prevalence of 16.6%. Chen et al. [16] evaluated 102 PCOS patients and reported the prevalence of IGT was 20.5% and that of non-insulin-dependent diabetes mellitus (NIDDM) was 1.9%. Lee et al. [17] reported that in Korean women with PCOS, the prevalence of IGT was 17.0% and the prevalence of type 2 diabetes was 1.0%. Cheung et al. [13] reported the prevalence of MetS in Hong Kong Chinese women with PCOS increased from 16.7% at under 30 years of age to 53.3% at over 40 years. According to previous publications, the presentation of insulin resistance in women with PCOS also demonstrated ethnic variance. In comparison with Caucasian women with PCOS, Eastern Asian women with PCOS had a lower prevalence of IGT and insulin resistance [14151618].
Inappropriate gonadotropin secretion in PCOS
Inappropriate gonadotropin secretion, especially an increased mean luteinizing hormone (LH) level, is a very common finding in women with PCOS [19]. The gonadotropin profile in women with PCOS is characterized by increases in serum levels of LH and in the LH to follicle stimulation hormone (FSH) ratio [20]. Although the LH level and the LH to FSH ratio are not required for diagnosis [1], disturbances of the hypothalamic-pituitary-ovarian axis are still thought to have a key etiologic role in PCOS patients [19]. The role of the LH to FSH ratio level in identifying women with PCOS remains controversial [212223], because measuring gonadotropin levels in women with PCOS has produced varied results under different conditions. Obesity is an important factor that may influence the LH to FSH ratio in women with PCOS. Arroyo et al. [24] reported that the mean 24-hour LH levels of non-obese women with PCOS were greater than in healthy controls in 95% of cases, whereas mean 24-hour LH levels failed to discriminate PCOS from healthy control women in 43% of obese women with PCOS. Our studies have found that obese women with PCOS have significantly lower LH to FSH ratios than non-obese women with PCOS, and, furthermore, that BMI is negatively correlated with LH in women with PCOS [25].
We suggest that in Asian women, a single measurement of the blood LH to FSH ratio, with proper sampling dates, is a valuable tool for evaluating PCOS. To diagnose PCOS in Taiwanese women, the LH to FSH ratio could be treated as an adequate marker of PCOS [25]. Further analysis of the decision thresholds of the LH to FSH ratio in PCOS diagnosis shows that an LH to FSH ratio of >1 results in the best combination of sensitivity and specificity [25]. We have found that the LH to FSH ratio is a good diagnostic indicator for women with chronic anovulation [25]. As for setting the decision threshold in the diagnosis of oligomenorrhea or anovulation, an LH to FSH ratio of >1 provides the best combination of sensitivity and specificity [25].
Obesity and PCOS in Taiwanese women
Obesity is a prominent feature of PCOS, occurring in 40% to 50% of patients [526]. Obesity appears to exert an additive, synergistic impact on the manifestations of PCOS, independently and negatively affecting insulin sensitivity, the risk of diabetes, and the cardiovascular profile [2728]. The prevalence of obesity in PCOS patients is greater than that in the general female population. Conversely, the prevalence of PCOS is greater in overweight and obese women than in lean women [29]. Obesity exacerbates both biochemical HA and chronic anovulation, which are the two most important diagnostic criteria for PCOS [29]. In a study of women with PCOS, BMI had a significant positive correlation with serum total testosterone and a negative correlation with serum LH [30]. Obese subjects with PCOS had a higher risk of developing Oligo-An (odds ratio [OR], 2.2; 95% confidence interval [CI], 1.3-3.7) and biochemical HA (OR, 2.6; 95% CI, 1.6-4.2) than non-obese women with PCOS [30]. Moreover, obese women with PCOS had significantly higher serum total testosterone levels, and more prolonged intervals between menstruation than non-obese women with PCOS [30]. Notably, obese women with PCOS presented with less acne than non-obese subjects (OR, 0.5; 95% CI, 0.3-0.9) [30]. We recently studied 273 women with four phenotypes of PCOS and found the insulin sensitivity index and the percentage of insulin resistance were not significantly different among the discrete phenotypes of PCOS and normal controls. However, obesity (OR, 14.0; 95% CI, 7.5-26.5) results in a higher risk for developing insulin resistance than HA (OR, 2.1; 95% CI, 1.3-3.6), Oligo-An (OR, 1.8; 95% CI, 1.0-3.3), and PCOM (OR, 1.4; 95% CI, 0.8-2.7) [31]. In comparison with HA, Oligo-An, and PCOM, obesity was the most important risk factor in determining insulin resistance in women with PCOS. The average BMI was much lower in Taiwanese PCOS women than Western women, which might partially explain the different clinical and biochemical presentations between East Asian and Western women [512].
Changes in the PCOS phenotype with age
The clinical features of PCOS change from adolescence to postmenopause [32]. PCOS has potentially profound implications for women of reproductive age regarding anovulatory infertility and symptoms related to elevated androgen levels. In addition, older women are prone to significant health problems related to hyperin-sulinemia, with a high risk for diabetes and cardiovascular risk factors [6]. The comorbidities of PCOS over the lifespan of an affected woman may inform individual therapeutic strategies, which could prevent long-term chronic metabolic diseases [32].
1. PCOS diagnostic criteria change with age
The prevalence of the different diagnostic criteria of PCOS (HA, Oligo-An, and PCOM) changes with age [33]. The clinical presentation of Oligo-An varies by age, with amenorrhea and oligomenorrhea being common among adolescents [34], while menstrual cycles may become regular with age in women with PCOS [3536]. HA partially resolves before menopause in women with PCOS [37]. Ovarian volume and follicle number decrease with age in women both with and without PCOS [38]. Furthermore, aging may also be associated with a defect in insulin action [39]. Therefore, the clinical features and metabolic consequences of PCOS may vary with age [40], and these age-related changes may affect the observed incidence of PCOS [41]. To understand the age-related changes in the diagnostic criteria of PCOS, it is crucial to determine the complications of the related syndromes.
2. PCOM changes with age
PCOM is defined by ovarian volume (>10 cm3) and/or increased antral follicle count (AFC, ≥12 in one ovary) on ultrasonographic examination. PCOM is a common, age-dependent finding among ovulatory women [42]. In a study using data obtained from 58,673 observations of ovarian volume, there was a statistically significant decrease in ovarian volume with each decade of life from age 30 to age 70 [43]. Pavlik et al. [43] study demonstrated a stable ovarian volume up to age 35, a rapid decline between ages 35 and 55, and a very minor decline after age 55. The ovarian volume in women aged 25 to 51 years was reported to reflect the number of primordial follicles remaining, and ovarian volume measurement by transvaginal sonography may determine the ovarian reserve and reproductive age [44]. The great majority of follicles that disappear are lost by atresia, and the rate of loss accelerates in the last decade of menstrual life [45]. In a community-based study of 262 ovulatory women ages 25 to 45, Johnstone reported that the prevalence of PCO was 32% and decreased with age, but that the percentage of women with an AFC ≥12 decreased from 62% at 25 to 30 years old to 7% at 41 to 45 years old [42]. The age-related decrease in follicle number seems more dominant than the age-related decrease in ovarian volume [42].
3. Oligo-amenorrhea change with age
Several studies suggest that a gradual normalization of menstrual cycle abnormalities occurs in PCOS with increasing age [3641]. Anti-Müllerian hormone (AMH) levels indicate the number of the ovarian follicle pool and may be a useful marker of ovarian reserves [46]. We demonstrated that serum AMH levels were strongly correlated to the number of menstrual cycles per year [47], which could explain the tendency of women with PCOS to achieve regular cycles as they grow older [37]. Decreases in serum AMH levels with age could be explained by ovarian follicle loss with increasing age. In Carmina et al. [48] study of 54 anovulatory hyperandrogenic women with PCOS, after a 5-year follow-up, 20% anovulatory patients became ovulatory at a mean age of 42 years. Women with PCOS achieve regular menstrual cycles with age and the development of a new balance in the polycystic ovary, caused solely by follicle loss through the process of ovarian aging, can explain the occurrence of regular cycles in older patients with PCOS [36]. Although the observed differences are relatively small during reproductive years, these age-related changes in women with Oligo-An may affect the observed incidence of PCOS [41].
4. Hyperandrogenism change with age
Elevated serum concentrations of androgens are the most consistent biochemical abnormality and may be considered the hallmark of the PCOS syndrome [49]. An age-related decrease in androgen secretion, as in normal women, also occurs in women with PCOS. We studied 781 women and found that the prevalence of acne and hirsutism were both negatively correlated with age in women with and without PCOS [50]. Bili et al. [41] studied 472 oligo-amenorrheic infertile patients and found that age was inversely correlated with testosterone, androstenedione, and dehydroepiandrosterone (DHEAS). Moran et al. [51] studied 145 hyperandrogenic women and reported a negative association between DHEAS levels and age. In a longitudinal study of 193 women who were followed from a mean age of 22 years to a mean age of 43 years, an approximately 25% decrease in testosterone levels and an approximately 30% decrease in DHEAS were observed [48]. We demonstrated that all serum androgen markers (total testosterone, androstenedione, and DHEAS) were significantly negatively correlated with age, and furthermore, that the prevalence of acne and hirsutism decreased with advanced age, mF-G score, and serum DHEAS levels, and there was a significant negative association with age in women with and without PCOS [50]. Accordingly, the prevalence of both clinical hyperandrogenism and biochemical HA should decrease in women of advanced age.
5. Metabolic syndrome change with age
MetS and insulin resistance are major concerns among the long-term complications in women with PCOS. Age is also an important risk factor for developing metabolic disorders and insulin resistance. Aging may also be associated with a defect in insulin action that is manifested by decreased whole-body tissue sensitivity to insulin without a change in tissue responsiveness [39]. We reported that advanced age was associated with increased cholesterol, triglyceride, and low-density lipoprotein levels; furthermore, fasting glucose and two-hour glucose were both significantly correlated with age [50]. The National Cholesterol Education Program Adult Treatment Panel (NCEP ATP III; third report of The National Cholesterol Education Program) guidelines define MetS according to the following five parameters: waist circumference, fasting serum glucose, fasting serum triglycerides, serum high-density lipoprotein cholesterol, and blood pressure. Most of these parameters worsen with age. Therefore, it is logical that MetS and insulin resistance are also age-dependent.
6. Phenotypes change with age
We reported a cross-sectional study that included 453 women with PCOS and found that for the women with the fulfilled diagnostic criteria for PCOS, younger women had a significantly higher percentage of acne and hirsutism, a higher mF-G score, and lower cholesterol and triglycerides than older women. In contrast, older women had higher levels of obesity, lower levels of androgens, and higher levels of insulin resistance and metabolic disturbances than younger women [50]. Our results are confirmed by Panidis et al. [52], who studied 1,212 women with PCOS and found a progressive decline in circulating androgens with advancing age. Furthermore, patients 21 to 30 years old had lower plasma glucose and insulin levels, lower homeostasis model assessment of insulin resistance index, and lower BMI than patients 31 to 39 years old. Both our and Panidis et al. [52] studies found that the prevalence of PCOS phenotypes changed with age, with younger women with PCOS having more severe hyperandrogenism but lower insulin resistance and BMI than older women with PCOS [52]. Interestingly, serum testosterone levels were positively correlated with BMI [50], and the prevalence of hyperandrogenism was twice as high for obese women as for non-obese women [30]. To fulfill the diagnostic criteria, older women with PCOS had a higher prevalence of obesity than younger women [5052]; therefore, the decrease in insulin resistance during the reproductive life of women with PCOS appears to be mainly attributable to the increase in obesity [52]. However, this raises the question of whether younger hyperandrogenic PCOS women become older obese women with insulin resistance. This question cannot be answered by a cross-sectional study. Recently, Carmina et al. [48] published a large longitudinal study that included 193 women with PCOS, aged 20 to 25 years, who were diagnosed according to the Rotterdam criteria and followed at five-year intervals for 20 years. After 10 years, androgens decreased; at 15 years, waist circumference increased; and at 20 years, ovarian volume decreased. Serum LH and FSH decreased nonsignificantly, and the fasting insulin and quantitative insulin-sensitivity check index were unchanged. Eighty-five women (44%) were ovulatory at 20 years, and 18 women (8%) could no longer be diagnosed as having PCOS [48]. After 20 years of follow-up in women with PCOS, androgens and ovarian volume decreased and there were more ovulatory cycles, suggesting a milder disorder, whereas metabolic abnormalities persisted and waist circumference increased [48].
Advanced age is associated with decreased hyperandrogenism and increased metabolic disturbances in women with and without PCOS. Hyperandrogenism and chronic anovulation may be the major disturbances in younger women with PCOS; however, increases in body weight might contribute to insulin resistance and metabolic disturbances in later life.
Cardio-metabolic risk in younger women
MetS is a cluster of cardio-metabolic factors that predisposes individuals to diabetes and cardiovascular disease (CVD) [53]. Although MetS and CVD are major causes of mortality for women of advanced age, the risks of MetS and CVD in reproductive age women are not well understood. The early detection of individuals at high risk for MetS using accurate measures of insulin resistance could improve the detection and prevention of CVD and diabetes [54]. Although many aspects of CVD are similar in women and men, there is a growing body of evidence to support sex and gender dimorphisms in the prevalence, presenting symptoms, management, and outcomes of CVD [55]. The most well known correlation between MetS and reproductive disorders is in women with PCOS; this correlation is not found in men. Although studies of PCOS and metabolic complications have been widely reported, the understanding of the correlation between endocrine status and metabolic complications in reproductive-aged women remains limited and controversial [56575859].
Cutoff values for risk factors for MetS should be determined before diagnosis is made, and these definitions should reflect gender and ethnic differences. To avoid the bias of gender and ethnic variance, instead of using arbitrary cutoff values, we performed cluster analysis using ten cardiovascular and metabolic risk parameters and classified the study population into high- and low-risk groups [60]. We performed a retrospective study on 573 Taiwanese women of reproductive age, including 384 at low risk and 189 at high risk of cardio-metabolic disease [60]. Our results indicated that risk factors for metabolic disease are associated with a low age of menarche, high levels of high-sensitivity C-reactive protein and liver enzymes, and low levels of sex hormone-binding globulin. Overweight/obesity (OR, 11.2; 95% CI, 8.0-15.7), PCOS (OR, 1.6; 95% CI, 1.3-1.9), oligo/amenorrhea (OR, 1.3; 95% CI, 1.1-1.4), and hyperandrogenism (OR, 1.4; 95% CI, 1.2-1.6) were found to increase the risk of cardio-metabolic disease. However, hyperprolactinemia and premature ovarian failure were not associated with the risk of cardio-metabolic disease. Therefore, according to our studies, both PCOS and obesity increased the risks of MetS and CVD; however, the OR in high-risk women with PCOS was much lower than that for women who were overweight/obese [60]. Androgen excess in women may signal a risk for coronary artery disease [61]. However, the different types of androgens should be evaluated separately. Specifically, the serum total testosterone level and free androgen index, but not androstenedione or DHEAS, were associated with the risk of MetS and CVD. Our study showed that obesity should be the major impacting factor on reproductive women with a high risk of MetS and CVD. The association between obesity and a cluster of cardiometabolic risk factors is stronger in women than in men, and this gender-specific difference exists in younger but not in older individuals [62]. Obesity accounts for the maximum variance in clustering and appears to be a more powerful correlate of cardiovascular risk in children and adolescents [63].
Finally, we summarize our findings as follows:
Hirsutism has a lower prevalence in Taiwanese women with PCOS than in Western women.
Taiwanese women with PCOS have a lower BMI and lower prevalence of IGT than Western women.
The LH to FSH ratio is a valuable diagnostic tool in evaluating Taiwanese women with PCOS, especially in the diagnosis of oligomenorrhea or anovulation.
Obese women with PCOS have a higher risk of developing oligomenorrhea, amenorrhea, biochemical hyperandrogenemia, insulin resistance, and lower LH to FSH ratios than non-obese women with PCOS.
Age-related changes affect the incidence and complications of PCOS.
Hyperandrogenism and oligomenorrhea are predominant in younger women with PCOS, and obesity and metabolic disturbances are predominant in older women with PCOS.
Overweight/obesity is the major determinant of cardiovascular and metabolic disturbances in reproductive aged women.
Early menarche, high levels of inflammatory markers and liver enzymes, and low SHBG are associated with high cardiovascular and metabolic risk.
Oligomenorrhea, hyperandrogenism, and PCOS are associated with high cardiovascular and metabolic risk.
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.