The effect of embryo catheter loading technique on the live birth rate
Article information
Abstract
Objective
Embryo loading (EL) is a major step in embryo transfer (ET) and affect on the success of in vitro fertilization (IVF). This study aimed to compare the effect of two different EL techniques on the rates of pregnancy and delivery in IVF/ET cycles.
Methods
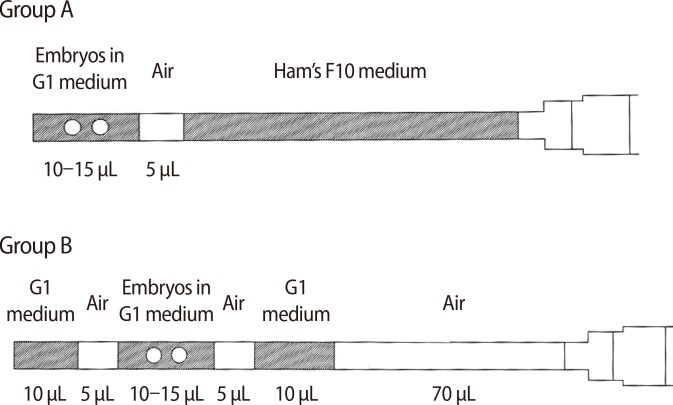
207 fresh ET and 194 Frozen-thawed ET (FET) cycles were included in this retrospective study. Two groups (A and B) were defined based on the EL technique used. In group A, the entire catheter was flushed with Ham's F-10 medium. The embryos were then drawn into the catheter using one air bracket. In group B, 70 µL of air was aspirated into the syringe and the catheter was flushed using Ham's F10 medium. The medium, air, embryos, air, and finally another layer of medium were then sequentially drawn into the catheter. The main outcome measures were the pregnancy and delivery rates.
Results
The groups did not differ with respect to the etiology of infertility, the source of spermatozoa, the quality of the embryos, the type of EL catheter, and the ease of transfer. The pregnancy rate was similar between two groups. In fresh ET cycles, a higher delivery rate was observed in group B than it group A (78.1% vs. 60%, p=0.1). In FET cycles, the rate of delivery was significantly higher in group B than in group A to a nonsignificant extent (88.9% vs. 58.8%, p=0.06).
Conclusion
EL techniques did not have a significant impact on the delivery rate in either fresh or FET cycles.
Introduction
Over recent decades, major progress has been achieved in many aspects of assisted reproductive technology (ART). Despite significant technological advances, in vitro fertilization (IVF) remains a complex process. The final, and perhaps the most crucial, procedure in IVF, embryo transfer (ET), has received less consideration than other techniques involved in IVF. Approximately 80% of patients undergoing IVF reach the ET stage, but the pregnancy rates remain low [123]. It has been well established that various factors, including embryo quality, and endometrial receptivity, as well as ET technique, can influence the implantation rate and the overall success of IVF [4].
The success of ART cycles depends on optimizing the efficiency of each procedure that is performed. It has been estimated that approximately 30% of all failures in ART may be due to poor ET technique [5]. Nevertheless, very few studies have reported ways of improving ART outcomes by optimizing the ET procedure. Therefore, it would be worthwhile to focus on maximizing the IVF success rate by standardizing the ET protocol. Despite the apparent simplicity of the ET procedure, it is a blind technique, the outcomes of which can be affected by many variables, including the presence of blood in the catheter [6], bacterial contamination of the catheter [7], the type of ET catheter [8], the presence of an air bubble in the catheter [9], the composition of the ET medium [10], and the volume of the transfer medium [11].
Embryo loading (EL) is a stage of the ET that is performed by a clinical embryologist. In general, two distinct catheter-loading methods exist: the air-fluid method and the fluid-only method [9]. The most commonly reported method has been found to be medium-air-embryo-air-medium with a prevalence of 42%, followed by medium in catheter with embryo, medium-air embryo, other methods, and finally medium with embryo with no air in between [3]. In a previous study, the ET medium volume was argued to be a main predictor for subsequent success in IVF/ET cycles [12]. In order to improve the state of the ART in embryo catheter loading and to identify the most efficient EL technique, we designed this retrospective study to evaluate the impact of two different embryo catheter loading techniques on pregnancy and delivery rates. We followed the patients until delivery and live birth in order to maximize the applicability of our conclusions.
Methods
1. Patient selection
A total of 401 cases met the inclusion criteria for this retrospective study. Of these cases, 207 were fresh ET cycles and 194 were frozen-thawed embryo transfer (FET) cycles. The study period was from October 2014 to March 2015. The couples were classified based on whether they experienced male factor infertility, female factor infertility, both male and female factor infertility or unexplained infertility. Egg donation, surrogacy, and in vitro maturation cycles were excluded from this study. Cycles were also excluded, if the ET record was not complete or the patient was lost to follow-up regarding the eventual occurrence of pregnancy or delivery. This study was approved by the ethics committee of the Yazd Research and Clinical Center for Infertility. Written informed consent was obtained from all patients.
2. Controlled ovarian hyperstimulation
Both a standard gonadotropin-releasing hormone (GnRH) agonist protocol and a standard GnRH antagonist protocol were used for ovarian hyperstimulation. GnRH agonist (Diphereline S.R. 3.75 mg; Ferring, Germany) downregulation was applied in the mid-luteal phase of a spontaneous menstrual cycle. Gonadotropin stimulation was initiated on day two of the cycle by administering human menopausal gonadotropin (Merional, IBSA, Lugano, Switzerland). In the antagonist protocol, 150 IU/day of follicle-stimulating hormone (Gonal F, Serono, Geneva, Switzerland) was administered on day two of the menstrual cycle. When at least one follicle reached 14 mm, 0.25 mg of a GnRH antagonist (Cetrotide, Merck Serono, Darmstadt, Germany) was initiated and continued until the day of human chorionic gonadotropin (hCG) injection. When at least two follicles measuring at least 17 mm were detected by ultrasonography, 10,000 IU of hCG (Pregnyl, NV Organon, Oss, The Netherlands) was injected for the final maturation of oocytes. Ovarian puncture and oocyte retrieval were performed 34 to 36 hours after the hCG injection.
3. Preparation of the endometrium
For luteal phase support in the agonist and antagonist protocols, the patients received a 400 mg progesterone suppository twice a day (Aburaihan Co., Tehran, Iran) and 2 mg of progesterone plus estradiol twice a day (Aburaihan Co.). Progesterone and estradiol were started on the day of oocyte retrieval and continued until the tenth week of gestation. In FET cycles, 6 mg of oral estradiol valerate (Aburaihan Co.) was administered daily, starting on the second day of the menstrual cycle. The endometrial thickness was checked by vaginal sonography, and when the thickness reached 7 mm or more, 100 mg of progesterone in oil (Aburaihan Co.) was intramuscularly administered.
4. IVF and intracytoplasmic sperm injection procedures
Semen analysis was performed according to World Health Organization guidelines [13]. Direct swim-up and density gradient centrifugation techniques were applied as appropriate for sperm preparation [14]. The type of ART was selected for each case based on the etiology of infertility, the maternal age, sperm parameters, and oocyte quality [1516].
5. Evaluation of fertilization and embryo morphology
The oocytes were checked 16 to 18 hours after injection and 18 to 20 hours after conventional IVF for the presence of two polar bodies and two pronuclei. The morphology of the embryos was evaluated two days before ET as described elsewhere [217]. Grade A and B embryos were considered high-quality embryos, whereas grade D embryos were not transferred.
6. Embryo freezing and thawing procedures
For vitrification, the embryos were suspended in an equilibration solution containing 7.5% ethylene glycol (EG) and 7.5% dimethylsulfoxide (DMSO) for 5 minutes at room temperature, then transferred to a vitrification solution containing 15% EG, 15% DMSO, and 0.5 M sucrose for 40 to 60 seconds. The embryos were loaded on to a Cryotop strip (Kitazato Co., Fuji, Japan) and were immediately plunged into liquid nitrogen.
The Cryotop strip was warmed by being directly inserted into the 4-(2-hydroxyethyl)-1-piperazine ethanesulphonic acid (HEPES)-based thawing medium with 1.0 M sucrose and 20% serum substitute supplement for 1 minute at 37℃. Warmed embryos were transferred to the diluent medium with 0.5 M sucrose for 3 minutes, then incubated in 0.25 M sucrose for 5 minutes and sucrose-free HEPES for 5 minutes. The embryos were subsequently placed in G2 medium and cultured for 2 hours before transfer [18].
7. Preparation of the ET dish
The day before ET, the inner well and outer well of a center-well organ culture dish (3037, Falcon, Santa Ana, CA, USA) were filled with 500 µL G1 medium (Vitrolife AB, Gothenburg, Sweden) and 3 mL of Ham's F-10 medium, respectively, and the dish was placed in a 37℃ incubator with 5% O2 and 6% CO2 overnight.
8. Embryo catheter loading technique
After confirmation of the patient's identity, the embryos selected for ET were transferred into the ET dish. The embryo catheter loading techniques were divided into two groups (A and B). In group A, the catheter was flushed with Ham's F-10 medium contained in a 1-mL airtight syringe. The embryos were then drawn into the ET catheter with one air bracket. In group B, 70 µL of air was aspirated into the syringe and the catheter was flushed with Ham's F-10 medium. The medium, air, embryos, air, and a final layer of the medium were sequentially drawn into the catheter (Figure 1).
9. ET technique
The ET procedure has been described in detail elsewhere [2]. Briefly, after inserting a sterile speculum into the vagina at the lithotomy position, the cervical mucus was aspirated using a Mucat tip (Laboratoire CCD, Paris, France). The clinician inserted the ET catheter 1.5 to 2 cm below the uterine fundus. The catheter was immediately checked for the presence of retained embryo(s), mucus, or blood inside or outside the catheter. Immediately after ET, the transfer was scored by the clinician as easy, moderate, or difficult. The number of procedures required for successful ET was also recorded [6]. A transfer was considered easy when the catheter penetrated the uterine cavity without cervical manipulation using forceps [19]. A transfer was considered difficult if blood or mucus was present in the catheter or if the catheter was inserted using forceps, and a transfer was considered moderately difficult if the catheter entered the uterine cavity without any difficulty, but was bloody.
10. Pregnancy outcomes
Chemical pregnancy was defined by a positive beta hCG test (30-100 mIU/mL; Monobind Inc., Lake Forest, CA, USA) 14 days after ET, and clinical pregnancy was verified by the detection of a fetal heartbeat at the end of the seventh week of gestation by ultrasonography. The clinical pregnancy rate was calculated as the total of clinical pregnancies divided by the number of chemically positive pregnancies. The implantation rate was calculated as the total number of intrauterine gestational sacs detected by ultrasonography divided by the total number of transferred embryos. The total number of clinical pregnancy losses before the twentieth week of gestation divided by the total number of chemical pregnancies was defined as the abortion rate. The live birth rate was defined as the total number of healthy newborns divided by number of chemically positive pregnancies.
11. Statistical analysis
Quantitative and qualitative data are presented as mean±standard deviation and percentages, respectively. The independent-samples t-test and the chi-square test were applied to the quantitative and qualitative data, respectively. All tests were two-tailed, and p-values <0.05 were considered to indicate statistical significance.
Results
No significant differences were found in the ages of the male and female partners between the patients who underwent fresh and FET cycles. A total of 401 patients were initially selected, but 27 cases were excluded because no information was present about clinical pregnancy and live birth, as the patients were lost to follow-up. A total of 2,147 oocytes in meiosis II were retrieved from patients, of which 1,090 were fertilized. A total of 464 and 443 day-two embryos were transferred in the fresh and FET cycles, respectively. Approximately 90% of the semen samples were collected by masturbation. The differences in the number of IVF and intracytoplasmic sperm injection cycles were insignificant between groups A and B (2.47% and 97.53% vs. 3.18% and 96.82%, respectively; p=0.7) (Table 1). No significant differences were found regarding endometrial thickness between groups A and B depending on whether fresh or FET samples were used (p=0.4 and p=0.1, respectively).

Comparison of clinical and laboratory characteristics between groups A and B in fresh embryo transfer cycles
Tables 1 and 2 compare the clinical and laboratory characteristics of groups A and B in fresh and FET cycles, respectively. No significant differences were found. We calculated the chemical pregnancy and implantation rates based on the cycles in which ET was performed. Moreover, the clinical pregnancy rate was calculated with regard to implantation-positive cycles. However, the clinical pregnancy outcomes of some patients were omitted because they were lost to follow-up.

Comparison of clinical and laboratory characteristics between groups A and B in frozen-thawed embryo transfer cycles
The clinical pregnancy and live birth rates showed no significant differences between groups A and B in either ET cycle (Tables 1, 2), although the rates of pregnancy and delivery tended to be higher in group B than in group A in fresh ET cycles (86.5% vs. 83.3%, p=0.7; 78.1% vs. 60%, p=0.1; respectively). In FET cycles, the delivery rate was also higher in group B than in group A (88.9% vs. 58.8%, respectively; p=0.06) (Table 2). One stillbirth occurred in the FET cycles, and the abortion rate did not differ between groups A and B in either the fresh or FET cycles (p=0.2 and p=0.1, respectively).
Discussion
Since ET technique is an important factor that influences ART outcomes, considerable attention and time should be devoted to this step. Despite the importance of ET, relatively few studies have focused on this technique since the introduction of IVF technology. The ET procedure can be separated into several parts, each of which may affect the success rate of ART cycles. One key step is embryo catheter loading, which involves several clinically relevant variables: the choice of a syringe [20], the volume of transfer medium [11], the concentration of proteins [21], the catheter loading speed [22], the viscosity of the transfer medium [23], and embryo placement in the catheter [24]. However, the volume of medium that is transferred and the presence of an air bubble are particularly controversial [2]. Moreno et al. [25] evaluated the effect of air loaded into the ET catheter on the success rate of ET. They did not find any difference with respect to the implantation and pregnancy rate. In contrast, another study demonstrated that air bubbles in the catheter along with a small volume of medium (<10 µL) had a negative effect on the implantation and pregnancy rates [11]. Recently, a systematic review showed that the use of air brackets was neither beneficial nor detrimental when compared to the fluid-only method of EL [9].
In this study, the different catheter EL techniques involved the air-fluid approach, since in both methods, the embryo-containing fluid was surrounded by air brackets. In group B, the embryos were set between two 5-µL air bubbles, whereas in group A, no air was placed in front of the embryos and the embryo-containing fluid was placed at the tip of the catheter. Some clinicians are of the opinion that the presence of an air bubble in the catheter can be useful for identifying the embryos and the medium during ultrasound-guided ET [26]. Additionally, the use of air bubbles around the embryo in the catheter, as in group B in our study, can protect the embryos from the cervical mucus and accidental discharge before entering the endometrial cavity [27]. However, others believe that even a small amount of air in the uterus could be a nonphysiological factor that has a deleterious effect on the embryos and on implantation [28]. Accordingly, a new variation of the EL technique has been suggested, in which only one air bubble is used at the tip of the ET catheter, thereby introducing a lesser amount of air into the uterine cavity [29]. It was found that the presence of air increased the likelihood of the embryo moving up towards the syringe, therefore increasing the risk of embryos being retained within the catheter [9].
Another controversial factor is the volume of the medium in the EL catheter. Improvements in ET procedures have led to the use of a small volume of medium (10-30 µL) for EL, because it has been proposed that ectopic pregnancy may occur due to high fluid volume in the ET catheter [30]. Moreover, it was found that the transfer of a high volume of medium can increase the chance of dislocation of the transferred embryos from the uterus into the cervix [31]. Montag et al. [32] compared the implantation and pregnancy rates associated with high and low fluid volumes (40-50 µL and 15-20 µL, respectively). They found that high fluid volume in the ET catheter increased both parameters. However, they used a high volume of fluid in combination with sequential culture media, unlike other studies.
In the present study, the total transfer volume differed between the two methods; it was greater in group B (35-40 µL) than in group A (15-20 µL). It has been hypothesized that a large volume of transfer medium and a large air interface may result in expulsion of embryos into the cervix [31] or ectopic pregnancy [30], thereby decreasing the pregnancy and implantation rates. However, our results did not show any significant differences in the pregnancy and implantation rates between groups A and B.
In order to identify the optimal ET procedure, more modifications in the EL technique and new EL methods are needed. With this in mind, we compared embryo catheter loading techniques involving loading directly from the culture micro drop versus loading from the transfer dish. We did not find any significant differences in the pregnancy rates between these two methods [2]. In this study, groups A and B did not differ with regard to transfer complications in fresh ET and FET cycles. Several studies have suggested that the ease of ET is strongly correlated to pregnancy outcomes. Despite the simplicity of this procedure, difficult transfers often occur, and it has been demonstrated that easy transfers and the absence of blood on the catheter increase the subsequent pregnancy rate [63334].
Another issue that is very important in ET procedures is whether the catheter is firm or soft. ET catheters should be sufficiently soft to avoid trauma to the endocervix or endometrium, and malleable enough to be easily directed into the uterine cavity. Several studies have demonstrated that soft catheters have the best results in terms of pregnancy rates [14]. Thus, in this study, we used soft catheters to minimize adverse outcomes. Additionally, embryo quality is one of the main factors affecting the ET success rate [2]. In our study, the rate of high-quality embryos did not differ significantly between groups A and B in fresh ET and FET cycles, as well as the other effective parameters such as the ages of the male and female partners, the causes of infertility, the type of stimulation protocol, and the number of transferred embryos (Tables 1, 2).
In conclusion, we found that the EL technique did not have a significant effect on pregnancy and live birth rates in either fresh ET or FET cycles.
Acknowledgments
The authors would like to thank Dr. Mostafa Omidi for his considerable help during the study.
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.