A high response to controlled ovarian stimulation induces premature luteinization with a negative impact on pregnancy outcomes in a gonadotropin-releasing hormone antagonist cycle
Article information
Abstract
Objective
The goal of this study was to investigate the relationship between serum progesterone (P4) levels on the day of human chorionic gonadotropin (hCG) administration and the pregnancy rate among women undergoing controlled ovarian stimulation for in vitro fertilization (IVF) or intracytoplasmic sperm injection-embryo transfer (ICSI-ET) using a flexible antagonist protocol.
Methods
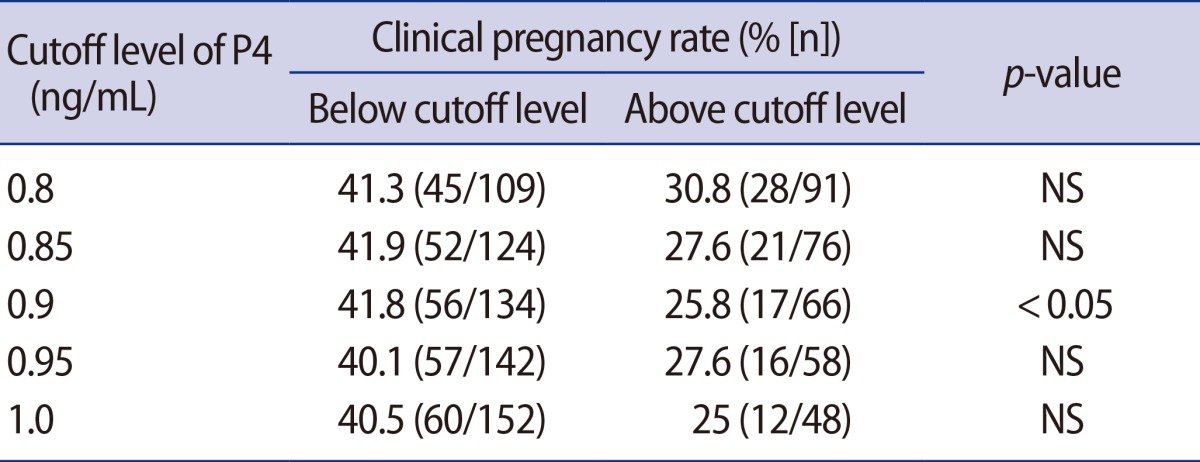
This prospective study included 200 IVF and ICSI-ET cycles in which a flexible antagonist protocol was used. The patients were divided into five distinct groups according to their serum P4 levels at the time of hCG administration (0.80, 0.85, 0.90, 0.95, and 1.00 ng/mL). The clinical pregnancy rate (CPR) was calculated for each P4 interval. Statistically significant differences were observed at a serum P4 level of 0.9 ng/mL. These data suggest that a serum P4 concentration of 0.9 ng/mL may represent the optimal threshold level for defining premature luteinization (PL) based on the presence of a significant negative impact on the CPR.
Results
The CPR for each round of ET was significantly lower in the PL group defined using this threshold (25.8% vs. 41.8%; p=0.019), and the number of oocytes retrieved was significantly higher than in the non-PL group (17.3±7.2 vs. 11.0±7.2; p=0.001). Elevated serum P4 levels on the day of hCG administration were associated with a reduced CPR, despite the retrieval of many oocytes.
Conclusion
Measuring serum P4 values at the time of hCG administration is necessary in order to determine the optimal strategy for embryo transfer.
Introduction
Controlled ovarian stimulation (COS) is an effective method of producing multiple oocytes during in vitro fertilization (IVF) procedures. COS-induced multiple follicular development affects steroidogenesis, resulting in a supraphysiologic hormonal milieu. According to the two-cell two-gonadotropin theory, follicle-stimulating hormone (FSH) acts on granulosa cells to promote the conversion of cholesterol to progesterone (P4), which is passed to the theca cells to be converted to androgens under the influence of luteinizing hormone (LH). Androgens are then passed to the granulosa cells to be converted to estradiol (E2) [1]. The elevated hormonal level during COS may be attributed to the excessive amount of growing follicles, which each produce a normal amount of hormones [2].
Elevated hormonal levels may contribute to changes in the endometrium, leading to endometrial advancement and embryo-endometrial asynchrony. One of the principal factors that may lead to endometrial advancement is protracted exposure to high E2 concentrations during COS. Multiple studies of IVF cycles have shown elevated E2 levels to be associated with lower pregnancy rates [34]. High E2 levels have been shown to have detrimental effects on endometrial receptivity in both agonist and antagonist cycles [5].
Mean serum P4 concentrations at the time of human chorionic gonadotropin (hCG) administration is lower in gonadotropin-releasing hormone (GnRH) antagonist cycles. The lower serum P4 levels in antagonist cycles may be explained by a less intense ovarian response to COS [6]. The ovarian response in antagonist cycles is lower than in agonist cycles, resulting in fewer growing follicles and the retrieval of fewer oocytes [789]. Therefore, the cutoff value for defining high P4 levels is different in agonist and antagonist cycles, and the threshold for antagonist cycles may be lower than for agonist cycles.
The importance of P4 levels at the time of hCG administration in antagonist cycles remains unclear. A meta-analysis has concluded that no evidence indicates that high serum P4 values have a negative impact on the implantation rate [2]. However, this conclusion has recently been challenged by other studies [10111213]. The majority of studies that failed to demonstrate an association between high P4 levels and the pregnancy rate used arbitrarily chosen P4 levels of 0.9 or 1.0 ng/mL to define premature luteinization (PL) [214]. Determining a threshold value using receiver operating curve analysis also may not be a suitable approach because the relationship between serum P4 levels and pregnancy outcomes is not linear.
In this study, we attempted to find the lowest P4 value that affected the clinical pregnancy rate (CPR) in antagonist cycles using trend analysis, and investigated whether PL had a clinically significant effect on the CPR. We also analyzed the effect of PL depending on E2 levels at the time of hCG administration in order to clarify the effect of PL on CPR.
Methods
A total of 200 embryo-transferred (ET) IVF cycle patients between May 2012 and July 2013 were included in this study. The inclusion criteria were being under 40 years of age, a normal basal hormona profile (basal FSH <10 mIU/mL and LH <10 mIU/mL), no diagnosis of polycystic ovarian syndrome, and unexplained primary or secondary infertility as the indication for IVF-ET. In all patients, serum P4 levels were determined at the time of hCG administration. The IVF cycles were divided into two groups according to the patient's P4 level.
COS began on the second day of the menstrual cycle, with daily injections of 225 or 300 IU of recombinant FSH (GONAL-F, Laboratorios Serono, Madrid, Spain; Puregon, Organon, Barcelona, Spain). Gonadotropin doses were adjusted according to the ovarian response, as reflected by the follicular number obtained via transvaginal ultrasound, and serum E2 levels. Highly purified human menopausal gonadotropin (Menopur, Ferring Pharmaceuticals, Geneva, Switzerland) was used if a poor response was anticipated.
A daily GnRH antagonist dose of 0.25 mg (Cetrotide, Merck Serono S.A., Geneva, Switzerland) was initiated based on a flexible protocol once a follicle became ≥12 mm in diameter, and was continued until hCG administration. When at least three follicles measured ≥18 mm in diameter, 10,000 IU of recombinant human chorionic gonadotropin (Ovidrel, Merck Serono S.A.) was administered. Oocytes were retrieved after 36 hours. ET was performed 3 days after oocyte retrieval. The ET number depended on embryo quality and patient age. Luteal support consisted of a daily 50-mg intramuscular injection of P4 (Progesterone in Oil, Watson Pharmaceuticals Inc., Parsippany, NJ, USA) from the day after oocyte retrieval until a serum pregnancy test.
Mature oocytes included grade I, I-1, and II oocytes. Clinical pregnancy was defined as the presence of a gestational sac after 5 weeks.
We categorized ovarian response into two groups according to the E2 level at the time of hCG administration: <3,000 pg/mL and ≥3,000 pg/mL group. This cutoff value was arbitrarily chosen. We explored the relationship between serum P4 levels and the CPR.
The relationship of the CPR to P4 concentration at the time of hCG administration is presented in Table 1 and Figure 1. The patients were divided into five distinct groups according to their serum P4 levels at the time of hCG administration: 0.80, 0.85, 0.90, 0.95, and 1.00 ng/mL. The CPR was calculated for each P4 interval. A reduction in CPR was observed with progressively higher P4 concentrations. This difference became statistically significant at the serum P4 level of 0.9 ng/mL. These data suggest that a serum P4 concentration of 0.9 ng/mL may represent the lowest level at which a significant negative impact on the CPR is observed.

Clinical pregnancy rate trend analysis at discrete serum progesterone (P4) cutoffs. Significance was evaluated using the chi-square test for each cutoff serum P4 level.
Serum P4 levels were measured at the time of hCG administration. The samples were tested with a microparticle enzyme immunoassay (AxSYM System; Abbott Cientifica S.A., Madrid, Spain), which had a sensitivity of 0.2 ng/mL. The intraobserver and interobserver variation coefficients were 9.6% and 3.9%, respectively.
We used the Student's t-test and the chi-square test for analyzing the characteristics and outcomes of the cycles. Univariate logistic and linear regression analyses were used to identify associations between and PL and other variables of interest. The p-values <0.05 were considered to indicate statistical significance. The statistical analysis was performed using SPSS ver. 14.0 (SPSS Inc., Chicago, IL, USA).
Results
The mean level of serum P4 at the time of hCG administration was 0.81 ng/mL (range, 0.02-3.56 ng/mL). Using trend analysis, a serum P4 level of 0.9 ng/mL was identified as the lowest level at which a detrimental effect on the CPR was found (Figure 1). Sixty-six patients (33.3%) had serum P4 levels above this threshold and were therefore defined as experiencing PL.
Table 2 summarizes the patient characteristics according to whether patients experienced PL. The E2 levels at the time of hCG administration and the number of oocytes retrieved were significantly higher in the patients who experienced PL (p<0.001).
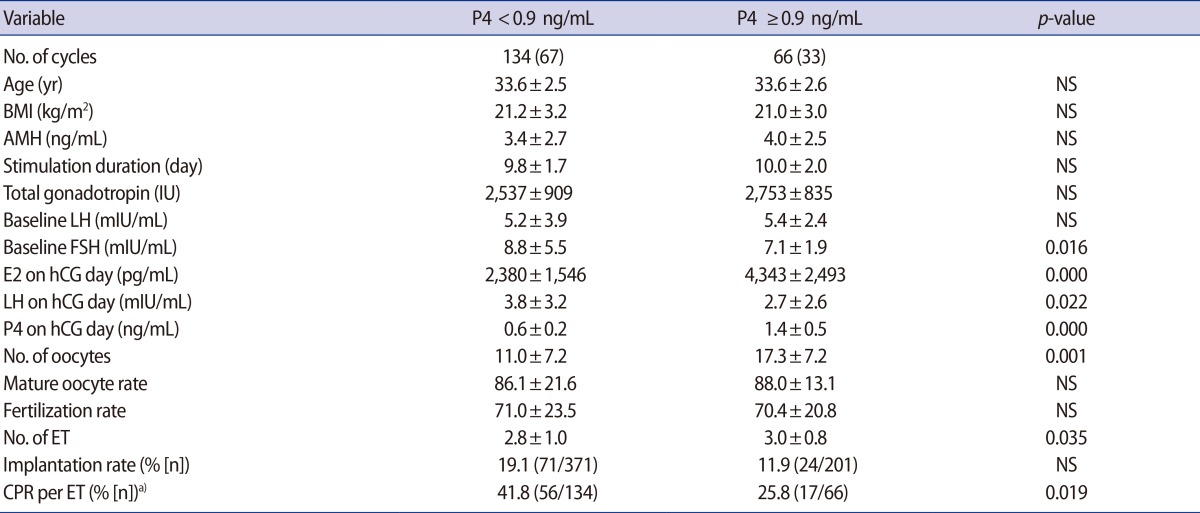
Demographics of patients and IVF outcomes between patients with and without premature luteinization, as defined by a progesterone cutoff value of 0.9 ng/mL
The quantity of ET procedures was significantly higher in the PL group (3.0±0.8 vs. 2.8±1.0; p=0.035), but the CPR for each instance of ET was significantly lower in the PL group (25.8% vs. 41.8%; p=0.019). The LH level at the time of hCG administration was significantly lower in the PL group (2.7±2.6 vs. 3.8±3.2; p=0.022) (Table 2).
Table 3 shows the effect of PL on the CPR depending on ovarian response. No differences in the CPR were found between the group in which the E2 level was <3,000 pg/mL and the group in which the E2 level was ≥3,000 pg/mL (37.7% vs. 34.3%; p>0.05).
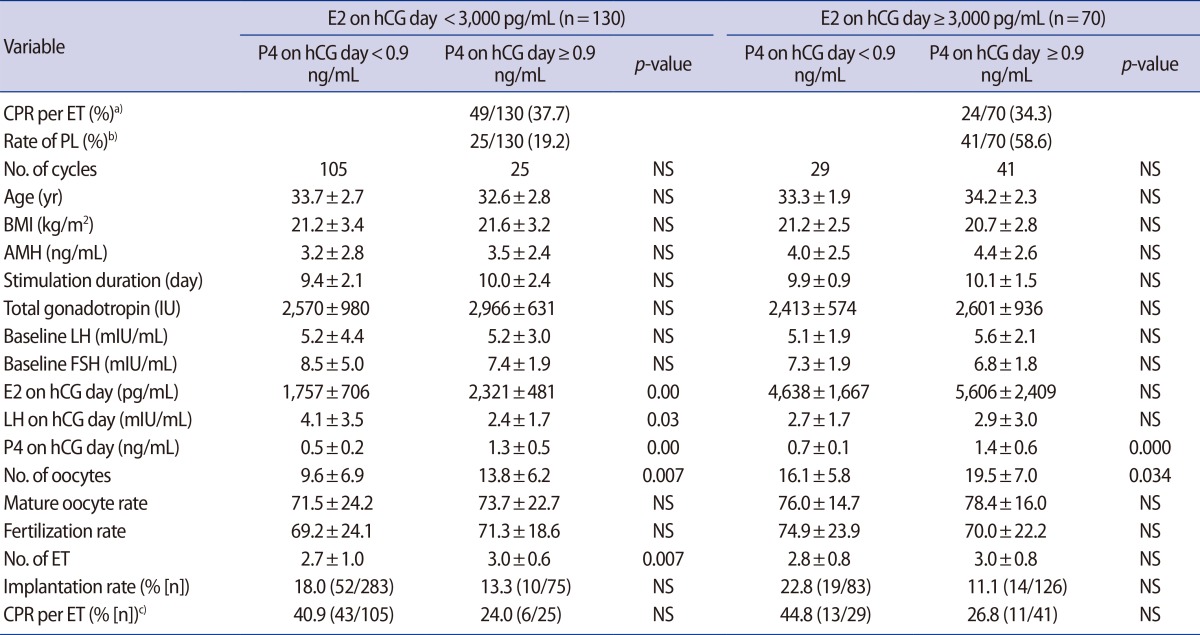
Subgroup analysis according to estradiol levels at the time of human chorionic gonadotropin administration
In group with E2 levels ≥3,000 pg/mL, the CPR was lower among patients who experienced PL than in the non-PL group (26.8% vs. 44.8%), but this difference was not statistically significant. In the low E2 group, the CPR was likewise lower in the PL group than the non-PL group (24.0% vs. 40.9%; p>0.05), although this difference was also not statistically significant.
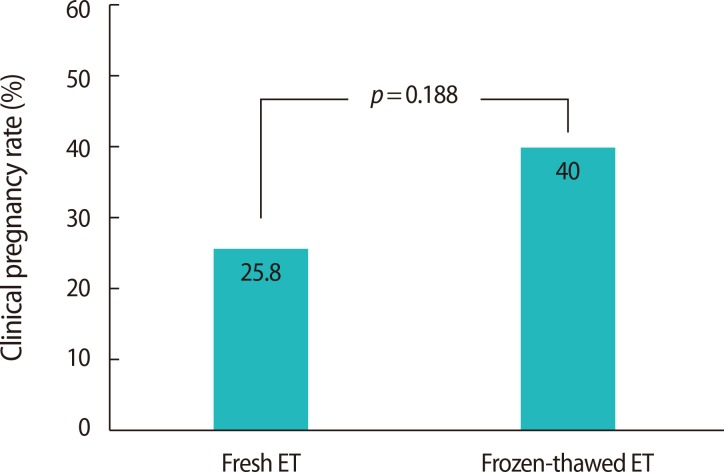
The P4 level at the time of hCG administration was related to the number of oocytes retrieved (r=0.383, p<0.001), the E2 level at the time of hCG administration (r=0.431, p<0.001), and the total gonadotropin dosage (r=0.186, p=0.021) (Figure 2). The CPR rate was 14% lower in the PL group as a whole in comparison to the patients who underwent a frozen-thawed ET cycle, which was performed in patients in the PL group who failed to achieve pregnancy in a previous cycle (25.8% [17/66] vs. 40.0% [10/25]) (Figure 3).

Regression analysis used to determine the serum progesterone (P4) threshold value. E2, estradiol; hCG, human chorionic gonadotropin.
Discussion
We demonstrated that PL was associated with a decreased CPR, despite a significantly increased number of retrieved oocytes and transferred embryos. Investigators have recently linked PL to multiple follicular development and increased ovarian steroidogenic activity [15]. During COS, P4 production is amplified in accordance with the number of follicles recruited and by FSH production. Our regression analysis also showed that the number of oocytes, dosage of FSH, and serum E2 levels at the time of hCG administration were the factors most closely related to the occurrence of PL, which also supports the above mechanisms. Therefore, when considering the phenomenon of PL, it would be reasonable to focus on the ovarian response, rather than on prematurely increased LH levels, as the primary pathogenic factor.
In this study, the LH level at the time of hCG administration was markedly lower in the PL group than in the non-PL group. This may have been due to negative feedback exerted by higher E2 levels on the pituitary LH in the PL group. This finding suggests that the primary source of PL is probably related to granulosa cell activity in growing follicles that are not luteinized, because the LH surge has been eliminated in 95% to 98% of patients since the introduction of GnRH analogs [1617]. Therefore, it is reasonable to conclude that the P4 level at the end of COS depends on ovarian response and tends to be higher in patients with a high ovarian response [18].
Serum E2 levels ≥3,000 pg/mL at the time of hCG administration and the presence of at least 15 retrieved oocytes had a predictive value at the P4 threshold value of 0.9 ng/mL. Thus, serum E2 levels ≥3,000 pg/mL and more than 15 retrieved oocytes, in combination with a threshold P4 level of 0.9 ng/mL, may be associated with PL in antagonist cycles. Sunkara et al. [19] demonstrated that the number of retrieved oocytes was closely correlated to the live birth rate, with an optimal number of 15.0, which is the same as the number of oocytes associated with a high risk of PL in this study.
Antagonist cycles have been found to lead to a lower ovarian response than agonist cycles, resulting in fewer follicles and the retrieval of fewer oocytes [789]. Therefore, P4 levels are different in antagonist and agonist cycles at the time of hCG administration [6]. In this study, the cutoff P4 value in antagonist cycles was determined to be 0.9 ng/mL using trend analysis. This method of determining a cutoff value is more reliable than doing so a priori because the relationship between serum P4 levels and pregnancy outcomes is not linear. Since many P4 assays are available, it cannot be excluded that different institutes may have different P4 thresholds.
The clinical consequences of PL were analyzed in fresh and frozen ET (FET) cycles to distinguish between endometrial factors and embryo quality. Although this finding was not statistically significant, the higher CPR in FET cycles suggests that the adverse effect of PL is exerted at the level of the endometrium rather than by affecting embryo quality. Recently, a retrospective analysis of 10,000 agonist cycles showed that the CPR in FET cycles was significantly higher than in fresh cycles at higher P4 concentrations [20]. On the basis of the FET results, it has been suggested that PL can lead to a change in the normal synchrony with the endometrium, and, when excessively disturbed, this asynchrony may reduce embryo implantation and pregnancy rates [21]. Therefore, avoiding fresh ET in such patients could be a promising way to prevent detrimental effects on the CPR.
Advanced endometrial maturation is present on the day of oocyte retrieval in IVF cycles. In the presence of extreme endometrial advancement, the CPR has been shown to be significantly decreased [2223]. Exposure to high E2 concentrations during COS has been considered the most likely mechanism of this early secretory transformation of the endometrium. It has been postulated that high E2 levels mediate the earlier expression of P4 receptors (PRs), which induce advancement of the endometrium in the late follicular phase [24].
However, according to our subgroup analysis of the E2 level at the time of hCG administration, E2 concentrations may not interfere with the CPR. High E2 and PL may be an independent predictors of the CPR. In COS with a GnRH antagonist, PR expression was significantly upregulated compared to natural cycles in both glands and the stroma, whereas estrogen receptors were down-regulated in the glands [25]. This finding was explained by the hypothesis that the increased E2 concentration during the follicular phase in COS upregulates PR expression in the endometrium. In the absence of E2 priming, P4 has no effect on the endometrium due to the lack of PR expression [26]. PRs may not be biologically active in the presence of PR upregulation until the P4 level reaches a certain threshold. PR exposure to the threshold quantity of P4 may be the crucial point leading to effects on the endometrium. It has been suggested that endometrium development in COS cycles is already altered at the time of hCG administration due to E2 elevation during COS. Then, if PL in the late follicular phase reaches the threshold value, PR activation occurs, resulting in endometrial advancement. Our data suggest that E2 and P4 exert distinct effects on endometrial advancement, although this possibility must be confirmed in prospective large-scale studies.
In conclusion, in GnRH antagonist cycles, PL is associated with a high response to COS and a decreased CPR.
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.