Oocyte cryopreservation for women with endometriosis: Justification, indications, and reproductive outcomes
Article information
Abstract
Women with endometriosis often experience diminished ovarian reserve and a decreased number of oocytes retrieved. This reduction is exacerbated after surgery. Nevertheless, oocyte quality does not seem to be compromised in these patients. When embryos of good quality are obtained, in vitro fertilization outcomes are generally satisfactory. Oocyte cryopreservation may represent a fertility preservation option for women with planned and/or prior surgery, as it enables the collection of oocytes in advance. Given the diverse manifestations of endometriosis, which vary by type, age, and ovarian reserve, the decision to pursue oocyte cryopreservation should be weighed individually. Moreover, the potential benefits of this approach on future fertility must be carefully considered. Considering current guidelines, the most appropriate candidates for oocyte cryopreservation among women with endometriosis are: patients with bilateral endometriomas, typically larger than 3 cm; those with prior surgery for unilateral endometrioma who exhibit ipsilateral or contralateral recurrence; and those with unilateral endometrioma on a single ovary. However, the size criteria for endometrioma warrant further discussion. Conversely, oocyte cryopreservation is inadvisable for patients: with unilateral endometrioma smaller than 3 cm and good ovarian reserve; who have undergone surgery for bilateral endometriomas, regardless of recurrence; and who have diminished ovarian reserve. While consensus indicates that decisions regarding diminished ovarian reserve should be individualized, fertility preservation should often be considered for patients with serum anti-Müllerian hormone levels below 0.5 ng/mL. In such cases, a prolonged duration may be necessary to retrieve the desired 10 to 15 oocytes.
Introduction
Endometriosis is a hormone-dependent disorder characterized by the presence of endometrial tissue outside the uterus, most commonly in the ovaries and pelvic cavity [1]. Its reported prevalence is between 10% and 15% among women of reproductive age and between 50% and 60% in women and adolescents with pelvic pain and/or unexplained infertility [2,3]. Known to cause a range of symptoms, including dysmenorrhea, dyspareunia, pelvic pain, painful defecation, and infertility, endometriosis meaningfully impacts quality of life in women of reproductive age [4].
Notably, infertility is strongly associated with endometriosis, with reports indicating that 30% to 50% of women with endometriosis experience this condition [5]. Numerous observational studies have demonstrated that endometriosis can adversely impact fertility. The prevalence of endometriosis is higher in women with infertility compared to their fertile counterparts, and those who are infertile are more likely to have moderate to severe forms of the disease [6,7]. A retrospective study revealed negative correlations between the severity of endometriosis and both clinical and ongoing pregnancy rates (PRs) [8]. Several mechanisms have been proposed to explain the impact of endometriosis on fertility, including alterations in anatomical structure, endometrial receptivity, and chronic inflammation. These changes may interfere with folliculogenesis, fertilization, implantation, or a combination of these processes [9].
Endometriosis triggers an inflammatory state through the activation of macrophages, T-cells, and natural killer cells, as well as the release of reactive oxidative species and pro-inflammatory cytokines. Secreted cytokines include interleukins, vascular endothelial growth factors, and tumor necrosis factors [10-14]. These inflammatory mediators adversely impact follicles, oocytes, embryos, and endometrial receptivity, ultimately exerting negative effects on fertilization and implantation [15-19].
In this review, we discuss the impact of endometriosis and its surgical treatment on ovarian reserve, the response to ovarian stimulation, and the clinical outcomes of in vitro fertilization (IVF). We then address the justification and indications for oocyte cryopreservation, as well as the reproductive outcomes of women with endometriosis following cryopreservation.
Ovarian reserve, response to ovarian stimulation, and clinical IVF outcomes in women with endometriosis
As indicated by a meta-analysis of 17 studies, women with endometrioma have significantly lower serum anti-Müllerian hormone (AMH) levels compared to those without the condition [20]. Furthermore, serum AMH levels are significantly lower in women with bilateral endometriomas than in those with unilateral endometrioma [21]. A recent retrospective study demonstrated that both serum AMH level (2.47 ng/mL vs. 3.32 ng/mL, p=0.001) and antral follicle count (8.8 vs. 12.3, p=0.001) were significantly lower in women with endometrioma compared to those without this condition [22]. The presence of endometrioma can lead to adverse effects on the surrounding healthy ovarian tissue, including increased oxidative stress and fibrosis, loss of cortex-specific stroma, smooth muscle cell metaplasia, reduced microvessel density, and impaired follicular maturation, and ultimately contributing to a decrease in follicular density. Cell damage mediators, proteolytic enzymes, and inflammatory molecules sequentially inflict damage on neighboring cells, resulting in decreased serum AMH levels [23].
Regarding IVF cycles, one meta-analysis showed that in women with endometrioma, the mean number of oocytes retrieved was significantly lower (mean difference, −0.23; 95% confidence interval [CI], −0.37 to −0.10) and the cycle cancellation rate significantly higher (odds ratio, 2.83; 95% CI, 1.32 to 6.06) compared to those without endometrioma [24].
A recent retrospective study also indicated that women with endometrioma required higher doses of gonadotropins during ovarian stimulation, and the number of oocytes retrieved was significantly lower (8.3 vs. 10.3, p=0.005) than that of patients without endometrioma [22]. Despite the lower number of oocytes retrieved in women with endometrioma, fertilization rates were similar between the two groups (83.3% vs. 85.3%), as were the rates of blastocyst formation (28.5% vs. 27.8%) [22].
Similarly, in a retrospective study, although the number of oocytes retrieved was significantly lower in patients with bilateral endometriomas compared to control participants, fertilization rates were similar between the two groups (67% vs. 70%). Additionally, the proportion of top-quality embryos per oocyte was the same (33% vs. 33%) [25]. The study also found comparable outcomes for implantation rates (22% vs. 23%), clinical PRs (39% vs. 37%), and live birth rates (LBRs; 29% vs. 33%) between the groups [25]. These findings suggest that while oocyte quantity may be reduced in women with endometrioma, oocyte quality appears to be unaffected.
Recently, Kamath et al. [26] conducted a retrospective comparison of IVF outcomes between women with endometriosis who underwent donor oocyte cycles and those who experienced autologous IVF cycles. The objective was to determine the impact of endometriosis on oocyte quality and LBR. When considering both fresh and frozen embryo transfer cycles, the LBR per cycle was similar between the two groups, even after adjusting for confounders. This result implies that endometriosis may have no more than a minimal negative impact on oocyte quality [26].
In symptomatic women with ovarian endometrioma, surgery is typically advised, with ovarian cystectomy being the preferred method due to its favorable postoperative outcomes regarding recurrence and spontaneous pregnancy [27,28]. However, cystectomy often results in ovarian damage and diminished ovarian reserve (DOR). In one study, serum AMH levels were reduced by 39.5% in women with unilateral endometrioma and by 57% in those with bilateral endometriomas at 9 to 12 months following ovarian cystectomy [29].
Numerous reports have indicated that surgery-related damage to the ovarian reserve can adversely influence IVF outcomes [24,30-32]. Demirol et al. [33] reported that relative to expectant management, surgical intervention may reduce ovarian responsiveness prior to IVF in women with endometrioma. Typically, poor IVF outcomes are attributed to a low number of oocytes retrieved post-surgery. In one evaluation of the effects of prior endometrioma surgery, the group that had undergone surgical treatment exhibited significant reductions in serum AMH, antral follicle count, ovarian sensitivity index, number of follicles, mature oocytes, fertilized oocytes, and embryos in comparison to the non-operated group. However, the fertilization rate, implantation rate, and cumulative LBR were comparable between groups [22].
Justification and indications for oocyte cryopreservation in women with endometriosis
Fertility preservation involves retrieving and storing oocytes or embryos, thus enabling a woman to have biological children at a later time [34]. Oocyte cryopreservation has been a clinically established method for preserving female fertility since the American Society for Reproductive Medicine (ASRM) lifted its experimental designation in 2013 [35]. Similarly, the European Society of Human Reproduction and Embryology (ESHRE) guidelines on fertility preservation, published in 2022, recommend offering oocyte cryopreservation as a standard option [36]. However, the practical benefits of oocyte cryopreservation for future fertility in women with endometriosis remain unclear.
The 2022 ESHRE guidelines recommend that clinicians discuss the advantages and disadvantages of fertility preservation with women who have extensive ovarian endometriosis. However, they also acknowledge that the benefits of fertility preservation in women with endometriosis are still uncertain [37]. Llarena et al. [38] have observed that providing a definitive indication for fertility preservation is challenging due to the limited research on the subject for this patient population.
If surgery is necessary, methods that are less harmful to ovarian function are recommended [39]. These approaches should be preferentially employed [40]. Indeed, some experts have recommended surgical drainage or ablation over cystectomy prior to ovarian stimulation in cases of large ovarian endometrioma that could interfere with oocyte retrieval [41]. However, no consensus has been reached on the best protocol for surgical drainage/ablation before ovarian stimulation; some clinicians favor simple drainage with the addition of gonadotropin-releasing hormone (GnRH) agonists, while others support drainage followed by sclerotherapy.
In some cases, it may be advisable to avoid surgery, particularly when the preservation of ovarian function is a concern. The 2022 ESHRE guidelines for endometriosis recommend against the excision of small endometriomas prior to IVF, especially in the context of repeated surgery or bilateral endometriomas [37].
Moreover, an increasing number of cases involve medical treatment without the need for surgery [42]. To manage the pain associated with endometriosis, the 2022 ESHRE guidelines recommend a range of hormonal treatments, including combined oral contraceptives, progestogens, and GnRH agonists [37]. However, reports have indicated that serum AMH levels can decrease spontaneously, even without surgical intervention [20]. Additionally, some research has indicated that medical treatment does not prevent the spontaneous reduction of AMH levels [43].
Preoperative oocyte cryopreservation is commonly undertaken in assumption that endometrioma is the most apparent benign cause of postoperative DOR. Nevertheless, the necessity of this procedure in all women with endometriosis is debatable, given the lack of a reliable method to predict a decline in ovarian reserve following surgery.
Strategies used for preoperative oocyte cryopreservation in unmarried or unpartnered women with endometrioma may differ markedly from those employed for women with cancer. Among unmarried or unpartnered women with endometriosis who opt for oocyte cryopreservation before surgery, four scenarios are possible: (i) they remain unmarried or unpartnered; (ii) they marry or having a partner and conceive naturally; (iii) they marry or having a partner but are unable to conceive naturally, leading them to attempt IVF with a sufficient number of high-quality oocytes retrieved; or (iv) they marry or having a partner but are unable to conceive naturally, and upon attempting IVF, they find that the quantity and/or quality of retrieved oocytes is low. Of these, the use of cryopreserved oocytes becomes unnecessary in scenarios (i), (ii), or (iii). However, cryopreservation would be essential in situation (iv).
In the case of (iii), cryopreserved oocytes may be preferred over fresh oocytes. This decision should be made in consultation with the woman. To date, no reports are available recommending the use of fresh versus frozen oocytes.
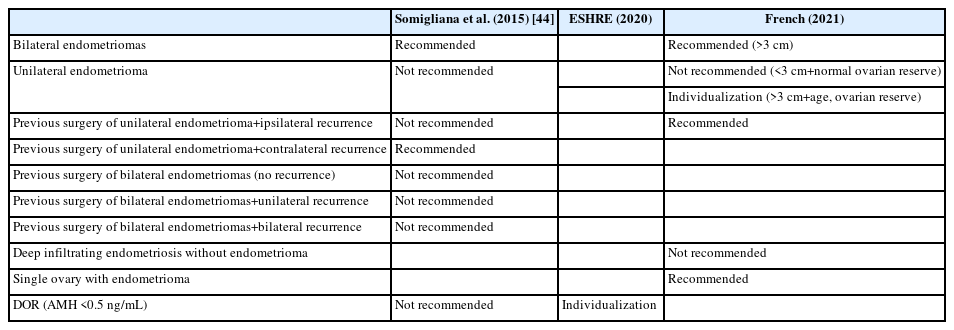
Somigliana et al. [44] categorized endometrioma into eight distinct clinical scenarios: (1) bilateral endometriomas; (2) unilateral endometrioma; (3) deep infiltrating endometriosis without endometrioma; (4) previous surgery for unilateral endometrioma with ipsilateral recurrence; (5) previous surgery for unilateral endometrioma with contralateral recurrence; (6) previous surgery for bilateral endometriomas without recurrence; (7) previous surgery for bilateral endometriomas with unilateral recurrence; and (8) previous surgery for bilateral endometriomas with bilateral recurrence (Table 1) [44].
These authors suggested considering three aspects before opting for oocyte cryopreservation: (i) Will a woman’s ovarian reserve decrease if she undergoes surgery? (ii) How many oocytes will likely be retrieved if oocyte cryopreservation is performed before surgery? and (iii) If preoperative oocyte cryopreservation is performed, is there a possibility of using cryopreserved oocytes in the future?
Given these three considerations, Somigliana et al. [44] suggested that the most valid indications for oocyte cryopreservation would be in the cases of (1: bilateral endometriomas) and (2: previous surgery for unilateral endometrioma with contralateral recurrence).
The remaining six scenarios were described as less compelling indications for oocyte cryopreservation. For instance, in cases of unilateral endometrioma (scenario (2)), enough oocytes may be retrieved; however, the likelihood of reduced ovarian reserve after surgery is relatively low, and the probability of the patient utilizing the cryopreserved oocytes is correspondingly small. For scenarios (6), (7), and (8), which involve previous surgery for bilateral endometriomas with or without recurrence, the ovarian reserve is likely already compromised, and few oocytes would be retrieved. Therefore, the rationale for oocyte cryopreservation in these situations is weak.
In 2020, ESHRE developed clinical practice guidelines regarding fertility preservation. The recommendations for women with endometriosis are as follows (Table 1) [36]: (1) The relevance of ovarian reserve testing in guiding fertility preservation options or treatment decisions for patients with endometriosis remains inconclusive; (2) Clinicians should understand that in patients with endometriosis, the involvement of the ovaries and the radicality of surgery can impact the ovarian reserve, as indicated by AMH levels. However, the relevance of DOR to future fertility remains unclear; (3) For women with DOR according to the Bologna criteria, with an AMH level below 0.5 ng/mL, recommendations should be individualized. The benefits of fertility preservation in this context are uncertain.
The 2020 ESHRE guidelines do not specify when oocyte cryopreservation should be performed for women with endometriosis, but they do suggest that it is not advisable in cases of DOR [36]. This stance aligns with the suggestion made by Somigliana et al. [44]. Given that oocyte cryopreservation in the context of DOR is likely to yield only a limited number of oocytes, the benefit of this procedure for future pregnancy is uncertain.
The 2021 French clinical practice guidelines offer indications and clinical recommendations for fertility preservation in women of reproductive age with benign gynecologic diseases, including endometriosis and non-endometriosis conditions, as well as idiopathic DOR [41]. This document outlines the following indications for fertility preservation in women with endometriosis (Table 1): (1) Fertility preservation should be proposed for bilateral endometriomas larger than 3 cm; (2) Fertility preservation should not be suggested for an initial occurrence of unilateral endometrioma smaller than 3 cm in a woman whose ovarian reserve is normal for her age; (3) For an initial occurrence of unilateral endometrioma larger than 3 cm, clinicians should evaluate the need for fertility preservation on an individual basis, considering factors such as the patient’s age and ovarian reserve; (4) Discussing fertility preservation is recommended in cases of recurrent unilateral endometrioma; (5) Proposing fertility preservation is advised in cases involving endometrioma affecting a single ovary; (6) It is not recommended to suggest fertility preservation for cases of minimal to mild endometriosis that do not involve the ovaries; (7) When ovarian stimulation for fertility preservation is indicated in cases of endometrioma(s), it is advisable to take action to increase the number of cryopreserved oocytes, assuming the ovaries are readily accessible for retrieval; (8) When ovarian stimulation for fertility preservation is indicated in cases of endometrioma(s), drainage should be considered as a first-line intervention if the lesions are excessively large or if they obstruct access to the ovaries for oocyte retrieval.
To summarize the French clinical practice guidelines, fertility preservation is indicated for women with endometriosis in four circumstances: (i) bilateral endometriomas larger than 3 cm; (ii) a unilateral endometrioma larger than 3 cm (with the decision individualized based on the woman’s age and ovarian reserve); (iii) unilateral endometrioma affecting a single ovary; and (iv) recurrent unilateral endometrioma.
In contrast, fertility preservation is not recommended in two situations: (i) when a unilateral endometrioma is smaller than 3 cm and the ovarian reserve is appropriate for the patient’s age; and (ii) in cases of minimal to mild endometriosis.
In addition, if ovarian stimulation is scheduled and the endometrioma is so large that it interferes with oocyte retrieval, drainage of the endometrioma should be considered.
Reproductive outcomes after oocyte cryopreservation in women with endometriosis
Women who are planning a future pregnancy would benefit from access to practical data. This information could help them set clear expectations and make informed decisions. In a group of patients undergoing elective oocyte cryopreservation for various indications—most of whom had infertility—the overall LBR per vitrified-warmed oocyte was 6.4% [45]. The estimated LBR per vitrified-warmed oocyte, stratified by age at the time of oocyte cryopreservation, was as follows: 8.7% for women under 30 years; 8.2% for women aged 30 to 34 years; 7.3% for women aged 35 to 37 years; 4.4% for women aged 38 to 40 years; 2.5% for women aged 41 to 42 years; and 1.0% for women aged 43 to 44 years. According to that report, to achieve a 70% chance of one live birth, women aged 30 to 34 years would need to cryopreserve 14 mature oocytes, women aged 35 to 37 years would need 15 mature oocytes, and women aged 38 to 40 years would need 26 mature oocytes.
However, the Practice Committee of the ASRM has noted that limited data are available to determine the optimal number of oocytes for elective oocyte cryopreservation [46]. In a retrospective study of women with endometriosis, the survival rate of vitrified oocytes was found to be 83.2%, with a resultant cumulative LBR of 46.4% [47]. The article noted a negative correlation between age and cumulative LBR. However, both the number of vitrified oocytes and their survival were positively associated with the cumulative LBR; no significant impact of surgery on these outcomes was observed. The number of vitrified oocytes was significantly higher in the group that did not undergo surgery (mean, 6.2) compared to the group that did. This number was comparable between the unilateral and bilateral surgery groups, with means of 5.0 and 4.5, respectively. In women aged 35 years or younger, the average number of oocytes vitrified per cycle was 8.6 in the no-surgery group and 5.1 in the surgery group. The cumulative LBR per patient was 72.5% in the no-surgery group compared to 52.8% in the surgery group (p=0.001). However, for women older than 35 years, ovarian response and clinical outcomes were similar regardless of surgical status. Consequently, the authors recommended considering oocyte cryopreservation before surgery, particularly for younger women.
In a subsequent report by the same first author, the cumulative LBR increased with the number of vitrified oocytes used in women with endometriosis, reaching 89.5% with the use of 22 oocytes. Notably, younger women experienced better outcomes; the cumulative LBR for approximately 20 oocytes was 95.4% in the younger group compared to 79.6% in the older group [48]. The report also indicated that the cumulative LBR was 87.0% when 11 to 14 oocytes were used and 61.1% for 15 or more oocytes in women aged 35 years or younger. For women older than 35 years, the rates were 34.3% with 11 to 14 oocytes and 15.8% with 15 or more oocytes.
The 2020 ESHRE guidelines for fertility preservation highlight a woman’s age at the time of cryopreservation as a key factor associated with live birth [35]. When determining the requisite number of oocytes for fertility preservation, one should consider the LBR or cumulative LBR. To increase the LBR, multiple cycles may be needed to collect a sufficient number of oocytes, even among young women with endometriosis. Rangi et al. [40] recommended the cryopreservation of 10 to 15 oocytes for women aged 35 years or younger and over 20 oocytes for those older than 35 years to have a realistic chance of achieving one or more live births, drawing on findings from a study by Cobo et al. [47].
Henry et al. [49] reviewed eight prior studies and determined the number needed to treat (NNT) for women with endometriosis. They calculated an NNT of 16 for oocyte cryopreservation, indicating that for every 16 women with endometriosis who undergo oocyte cryopreservation, one would be expected to have a child. However, their analysis included only women under 35 years old. Consequently, the calculated NNT may not fully represent the effectiveness in relation to the LBR.
Conclusion and recommendations
In women with endometriosis, both ovarian reserve and the number of oocytes retrieved are reduced, even in the absence of surgery, and these measures are further diminished following surgical intervention [20,22,24,29-32]. Despite DOR or a decreased quantity of oocytes in women with endometriosis, the quality of the oocytes seems to remain unaffected [22,25]. Several studies have indicated that clinical PR, LBR, and embryo implantation rates are satisfactory when good quality embryos are obtained [22,25,26].
Thus, oocyte cryopreservation represents a potential fertility preservation strategy among women planning to undergo surgery (even those who have undergone previous procedures), to secure additional oocytes in advance. However, given the wide variety of endometriosis types and situations, as well as the variability in age and ovarian reserve, it is essential to assess the necessity of oocyte cryopreservation on a case-by-case basis. Moreover, the potential benefits of oocyte cryopreservation for a woman’s future fertility should be carefully evaluated.
Even when surgical treatment is considered, the approach that minimizes the risk of DOR should be prioritized, particularly for women who intend to conceive in the future [39,40]. If the preservation of ovarian reserve is a substantial concern, opting for expectant management or nonsurgical treatment may be a preferable alternative.
Based on the guidelines released to date, the most appropriate indications for oocyte cryopreservation in women with endometriosis include: (i) those with bilateral endometriomas, typically larger than 3 cm; (ii) those who have undergone surgery for unilateral endometrioma and have experienced ipsilateral or contralateral recurrence; and (iii) those with unilateral endometrioma affecting a single ovary. However, the French clinical practice guidelines specifically recommend oocyte cryopreservation for women with bilateral endometriomas larger than 3 cm, suggesting that further discussion is warranted regarding the size criteria for endometrioma.
Unilateral endometrioma larger than 3 cm presents an ambiguous situation. Nevertheless, oocyte cryopreservation may be recommended in cases of substantial concern for preserving ovarian reserve and a serum AMH level of at least 0.5 ng/mL. If ovarian stimulation is planned for oocyte cryopreservation and the endometrioma is large enough to impede oocyte retrieval, percutaneous drainage of the endometrioma should be considered.
However, oocyte cryopreservation is not recommended for certain individuals, including: (i) those with unilateral endometrioma smaller than 3 cm who display good ovarian reserve; (ii) those who have undergone previous surgery for bilateral endometriomas (irrespective of recurrence); and (iii) those with DOR. While the definition of DOR remains a subject of debate, it is advisable to provide individualized guidance on fertility preservation for women with a serum AMH level below 0.5 ng/mL, in accordance with the ESHRE guidelines. When serum AMH levels are low, accumulating the desired 10 to 15 oocytes can be a prolonged process.
If oocyte cryopreservation is planned, clinicians can inform patients of the probability of LBR using vitrified oocytes, as indicated by findings from two studies by Cobo et al. [47,48]. While research in this area is still limited, it suggests that cryopreserving 10 to 15 oocytes for patients aged 35 years or younger, and more than 20 oocytes for those older than 35, offers a realistic chance of achieving one or more live births in women with endometriosis [40].
Clinicians should inform patients that irrespective of surgery, natural pregnancy is possible without the use of vitrified oocytes, provided that the ovarian reserve is adequate and that no other infertility factors are present. Additionally, pregnancy through IVF can be achieved without vitrified oocytes if a sufficient number of fresh oocytes can be retrieved.
Notes
Conflict of interest
Byung Chul Jee has served as the editor-in-chief of Clinical and Experimental Reproductive Medicine since 2018. However, he did not participate in the selection, evaluation, or decision-making process for the peer review of this article. No potential conflicts of interest related to this article have been reported.
Author contributions
Conceptualization: SJC, BCJ. Methodology: SJC, BCJ. Formal analysis: SJC, BCJ. Data curation: SJC, BCJ. Writing-original draft: SJC, BCJ. Writing-review & editing: SJC, BCJ. Approval of final manuscript: SJC, BCJ.