Outcomes of female reproductive performance with assisted reproductive techniques after recent mild to moderate COVID-19 infections: An observational study
Article information
Abstract
Objective
Although several studies have investigated the clinical outcomes of assisted reproductive techniques (ARTs) and male reproductive performance during the coronavirus disease 2019 (COVID-19) pandemic, few well-designed studies have focused on female reproductive performance after a recent mild to moderate COVID-19 infection.
Methods
This cohort study of 75 participants was performed at Arash Women’s Hospital, Tehran, Iran. The 35 participants in the exposed group were women with infertility who visited the hospital from October 2021 to February 2022 and were prospectively followed. The 40 participants in the non-exposed matched group were selected retrospectively from hospital patients who underwent ART before the COVID-19 pandemic. Study variables were compared between the non-exposed and exposed groups.
Results
The exposed group had a non-significantly smaller total of retrieved oocytes and fewer mature oocytes than the non-exposed group (p=0.26, p=0.10). The numbers of total and transferred embryos were also lower in the exposed group, but these results were not statistically significant (p=0.27, p=0.77). The rate of spontaneous abortion was higher in the exposed group (43% vs, 11%), and the ongoing pregnancy rate was lower (57% vs, 89%); however, these differences were not statistically significant.
Conclusion
Recent mild to moderate COVID-19 infection seems to have no detrimental effects on female reproductive performance and embryo transfer outcomes; however, considering the small sample size of the present study, the statistically insignificant differences in the exposed group—namely, fewer embryos, fewer retrieved oocytes, a lower ongoing pregnancy rate, and a higher spontaneous abortion rate—might be important from a clinical standpoint.
Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is mainly associated with respiratory symptoms. In some cases, it causes severe inflammatory responses that may lead to acute pneumonitis, dyspnea, respiratory distress, and death [1,2]. Along with the negative respiratory effects of infection, both male and female reproductive tissues can be potential targets for SARS-CoV-2 because of the expression of transmembrane receptors for this virus, such as angiotensin-converting enzyme 2 and transmembrane serine protease 2 [3]. It is known that maintenance of the controlled microenvironment of follicular fluid, including the balance of proteins, metabolites, and cytokines, is critical for the retrieval of good-quality oocytes during assisted reproductive techniques (ARTs) [4,5]; however, in granulosa cell lines that were stimulated with follicular fluid from post-COVID-19 patients undergoing ART, the markers of DNA damage and inflammatory response due to viral infection were significantly higher [6]. Together with the direct effects of SARS-CoV-2 on female reproductive tissues through transmembrane receptors, COVID-19 infection may be able to affect female reproductive performance indirectly via vasoconstriction, inflammation, angiogenesis, oxidative stress, or apoptosis [7].
Several studies have evaluated the effects of COVID-19 on fertility and ART outcomes. Some assessed only the impact of the COVID-19 pandemic, regardless of infection status, whereas others focused specifically on the consequences of COVID-19 infection. According to our searches, many of these studies have focused on clinical outcomes such as pregnancy rate or male reproductive performance, and few well-designed studies have investigated female reproductive performance during the ART cycle. Despite comprehensive global programs against the pandemic and complete vaccination, people are still infected by SARS-CoV-2 [8]. Thus, in the present observational study, we aimed to evaluate female reproductive performance and ART outcomes after recent mild to moderate COVID-19 infections.
Methods
1. Design and settings
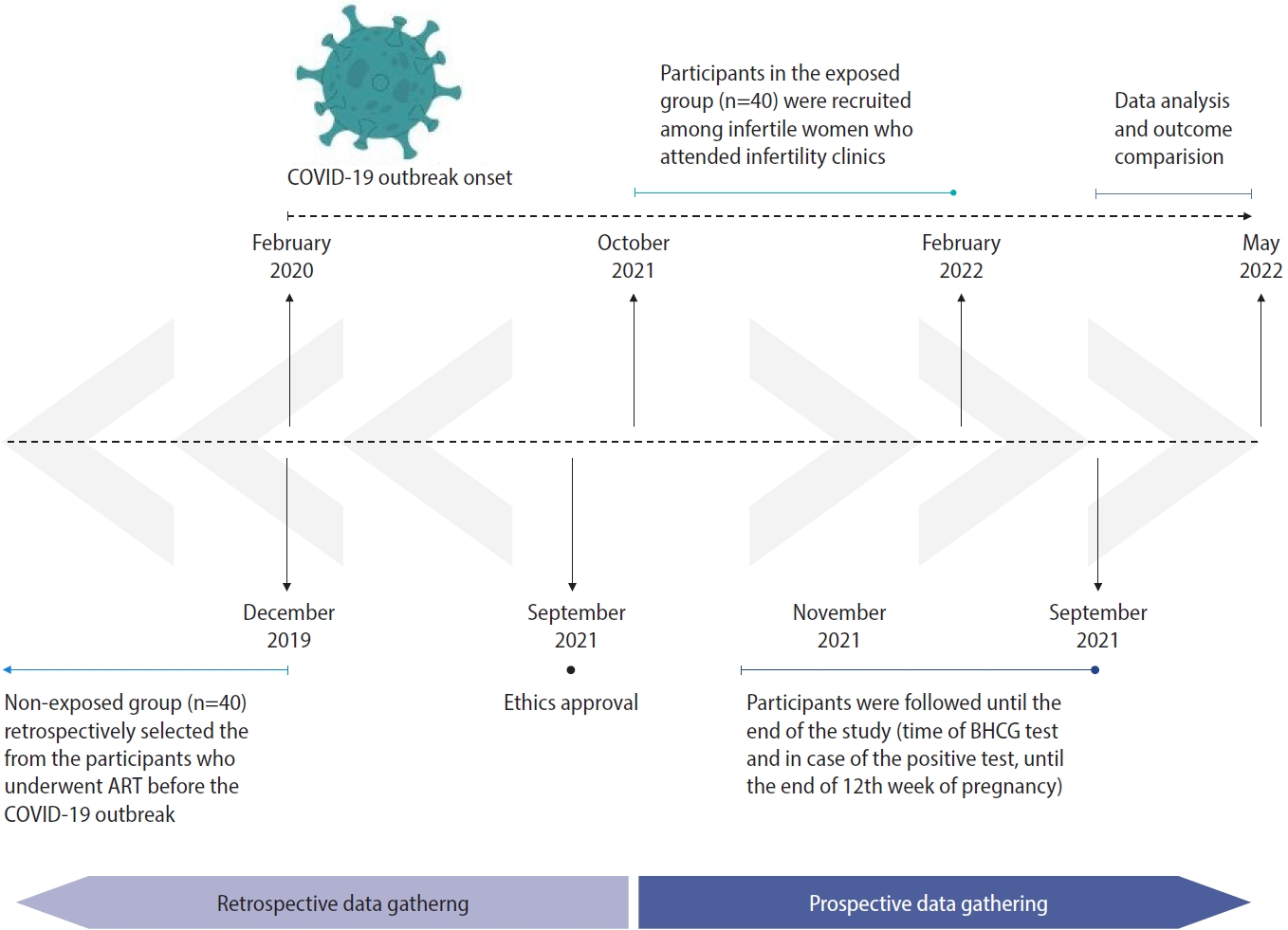
We employed an ambi-directional cohort design (retrospective and prospective) to assess the impact of recent COVID-19 infection on reproductive performance in women who underwent ART in an infertility clinic at Arash Women’s Hospital, Tehran, Iran, from October 2021 until May 2022. The study population is schematically presented in Figure 1.
2. Ethics
The study protocol was approved by the Research Ethics Committees of the School of Medicine, Tehran University of Medical Sciences, Tehran, Iran on September 8, 2021 (approval code: IR.TUMS.MEDICINE.REC.1400.642). Informed consent was obtained from all participants. The study was designed and performed in accordance with the Declaration of Helsinki.
3. Participants and study groups
Participants in the exposed group (n=40) were recruited from women with infertility who visited our clinic from October 2021 to February 2022. They were prospectively followed until the end of the study (time of beta-human chorionic gonadotropin [BHCG] test and, in case of a positive result, until the end of week 12 of pregnancy). Participants in the exposed group were included if they were 18 to 40 years old, underwent ART, and had a recent symptomatic COVID-19 infection; recency was defined as within the 3 months preceding the start of the ART cycle, considering the duration of folliculogenesis, and infection was determined according to recorded positive serology and polymerase chain reaction (PCR) tests. We excluded cases with asymptomatic or presymptomatic infection and cases with a history of severe or critical COVID-19 illness. Patients whose symptoms began during controlled ovarian hyperstimulation (COH) or study outcome assessments, or whose husbands had self-reported experiencing COVID-19 infection during the 3 months preceding ART, were also excluded. The classification of illness severity was based on the United States National Institutes of Health COVID-19 Treatment Guidelines [9]. Briefly, this guideline grouped adults with SARS-CoV-2 infection into categories of asymptomatic or presymptomatic infection and symptomatic infection associated with mild, moderate, severe, and critical illness. Accordingly, mild illness describes patients who have any of the signs and symptoms of COVID-19, but do not have dyspnea or abnormal chest imaging. Individuals who show evidence of lower respiratory disease (clinically or in imaging) and who have an oxygen saturation measured by pulse oximetry (SpO2) ≥94% are categorized as having moderate illness. Individuals with SpO2 <94%, a ratio of arterial partial pressure of oxygen to fraction of inspired oxygen <300 mm Hg, a respiratory rate >30 breaths/min, or lung infiltrates >50% are categorized as severe, and those with respiratory failure, septic shock, or multiple organ dysfunction should be considered as having critical illness.
We retrospectively selected the non-exposed group (n=40) from patients who underwent ART at Arash Women’s Hospital before December 2019, when SARS-CoV-2 and COVID-19 were first identified. The last patient included in the non-exposed group was seen at our infertility clinic in April 2019. The non-exposed group was selected randomly and was matched with the exposed group for age, duration, and cause of infertility. The causes of infertility included in our study were female factor, male factor, and unexplained. Subsequently, we subdivided the female factor into three categories: diminished ovarian reserve, anovulation, and tubal factor. We did not consider patients with mixed male/female factor infertility issues because they could introduce confounding and heterogeneity to our analysis.
4. Study outcomes and variables
The primary outcome of the present study was female reproductive performance during the ART cycle. The total number of retrieved oocytes and the number of mature oocytes (oocytes that reached the metaphase II stage of meiosis [M II oocyte]) were considered to be reproductive performance indicators. Secondary outcomes were the number of embryos, their developmental stages, and the rates of fertilization, implantation, chemical pregnancy, and clinical pregnancy.
The study variables were collected from gynecological and embryological records using the infertility clinic database and interviews with participants. We categorized the study variables as baseline (including demographic characteristics, age, and body mass index), obstetric history (gravid, parity, live child, and miscarriage), infertility history (infertility duration, previous failed ART cycles, and infertility type and cause), baseline hormonal profile (anti-Mullerian hormone [AMH], follicle-stimulating hormone [FSH], and luteinizing hormone [LH]), semen parameters before ART (semen volume, sperm concentration, and progressive motility rate), COH and ART cycle characteristics (COH regimen, developmental stage, and the number of transferred embryos), and outcome variables.
The chemical or pre-clinical pregnancy rate was defined as the ratio of the number of participants with a positive BHCG test to the total number of participants who underwent embryo transfer (ET). Pregnancies with a positive fetal heartbeat at ultrasound assessment were considered clinical pregnancies. We considered pregnancies lasting for more than 12 weeks to be ongoing pregnancies. Miscarriage was defined as an early spontaneous loss of pregnancy before the completion of the 12th week.
5. Statistics
To calculate the fertilization rate, the total number of zygotes with two pronuclei was divided by the total number of injected M II oocytes. The abortion rate was calculated by dividing the total number of miscarriages by the total number of pregnancies.
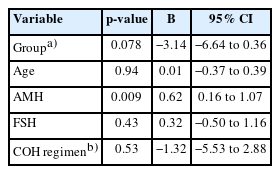
All analyses were conducted using SPSS Statistics for Windows version 23.0 (IBM Corp.). Continuous variables were reported as mean±standard deviation and were compared with a t-test. Discrete variables were declared as frequency (percentages) and were compared using the chi-square test or Fisher exact test for small numbers, as appropriate. A multivariate linear regression model was applied to identify the effect of COVID-19 exposure (group), age, AMH, FSH, and COH protocol on the total number of retrieved oocytes. All p-values were derived from two-tailed tests and considered significant at p<0.05.
Results
Finally, the study variables were compared between the 35 participants in the non-exposed group and 40 participants in the exposed group. Five of the 40 participants in the non-exposed group were lost to follow-up and excluded from the final analyses because of a lack of information about the ART or pregnancy outcome.
The baseline characteristics are detailed in Table 1. The mean age of participants was 31.61±4.81 years and 32.65±3.55 years in the non-exposed and exposed groups, respectively. No statistically significant differences were found between the groups for age (p=0.36) or obstetrics history. The groups also had similar infertility durations and previously failed ART cycles.
As has been shown, primary infertility was more prevalent than secondary infertility among participants who visited our clinic after the COVID-19 pandemic had begun (75% vs. 25%). In contrast, in the non-exposed group, whose members visited our clinic before the COVID-19 pandemic, about half of the participants had primary infertility, and the other half had secondary infertility (54% vs. 46%). This difference was statistically significant (p=0.04). Male factor infertility was the most prevalent cause of infertility in both groups. No significant difference was found between the groups regarding the cause of infertility (p=0.40).
The level of AMH was lower in the exposed than in the non-exposed group (3.00±3.51 vs. 4.35±3.88), although this finding was not statistically significant (p=0.15). No significant difference was found between the groups regarding FSH level (p=0.87). The level of LH was higher in the exposed group, but the difference was not statistically significant (p=0.06).
Semen analysis before the ART procedure showed no difference between the two groups regarding any of the sperm parameters. The COH protocol and ET characteristics were not different between the groups; the prevalence of fresh ET was higher in the exposed group (42.5%, compared to 26% in the non-exposed group), but the finding was not statistically significant.
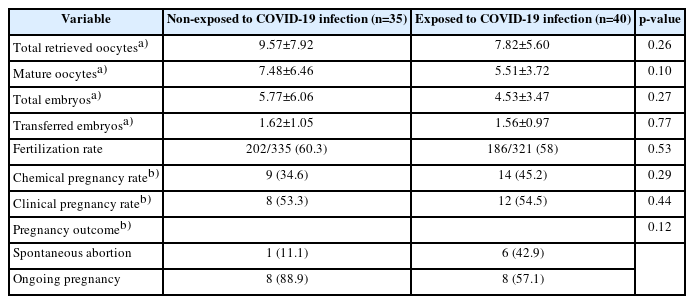
Table 2 shows the reproductive performance indicators. The exposed group had a lower number of both total retrieved oocytes and M II oocytes (7.82±5.60 and 5.51±3.72) than the non-exposed group (9.57±7.92 and 7.48±6.46); however, these findings were not statistically significant (p=0.26, p=0.10). The linear regression model for the total number of retrieved oocytes demonstrated no effect from COVID-19 exposure (p=0.07), age (p=0.94), FSH (p=0.250), and COH protocol (p=0.25) on the total number of retrieved oocytes, while AMH level was to be a significant factor (p=0.009) (Table 3).
The ART cycle and ET outcomes are described in Table 2. The total number of embryos and the number of transferred embryos were lower in the exposed group, but these results were not statistically significant (p=0.27 and p=0.77, respectively). Neither chemical nor clinical pregnancy rates had a statistically significant difference between the groups. Compared to the non-exposed participants, in the exposed group the rate of spontaneous abortion was higher (43% vs. 11%), and the ongoing pregnancy rate was lower (57% vs. 89%); however, these differences were not statistically significant.
Discussion
The present study showed that having experienced a recent COVID-19 infection had no detrimental effects on female reproductive performance and ET outcomes; however, the group of participants who had been exposed to COVID-19 had fewer whole embryos, transferred embryos, total retrieved oocytes, and M II oocytes, as well as lower ongoing pregnancy rates. According to the results of the regression analysis, we hypothesized that a smaller number of retrieved oocytes and, consequently, fewer embryos may be related to the lower baseline AMH level in the exposed group. We have no data about the exposed group’s pre-infection AMH levels. Unfortunately, the present study cannot determine with precision whether the exposed group's statistically insignificant lower level of AMH was a consequence of COVID-19 infection or due merely to more referrals of patients with diminished reproductive performance for ART.
Controversial studies have been published regarding the effects of COVID-19 infection on ovarian function and reserve. Herrero et al. [6] showed that patients with higher IgG levels against SARS-CoV-2 had lower numbers of retrieved oocytes. Madenli et al. [10] showed that 3 months after COVID-19 infection, AMH levels had decreased and menstrual cycle irregularity had increased. In a study by Li et al. [11], mean sex hormone levels and ovarian reserve were not altered significantly, while menstrual changes such as a smaller volume of vaginal bleeding or a prolonged cycle were reported in 20% of patients. These might be signs of transient sex hormone changes caused by a suppression of ovarian function, which returned to baseline levels soon after recovery from infection [11]. Kolanska et al. [12] concluded that a history of mild COVID-19 infection does not seem to alter the ovarian reserve as evaluated by AMH concentrations. However, a small number of participants with positive COVID-19 tests were assessed in their study (14 participants) [12]. Orvieto et al. [13] found similar results; however, their sample size was very small (nine participants).
In comparing the pregnancy outcomes of women who underwent ART before and after the COVID-19 pandemic had begun, a systematic review and meta-analysis of papers published in Chinese and English found no significant difference in the rates of clinical pregnancy and miscarriage. The review did not assess other ART outcomes such as oocyte yield, embryo quantity, or embryo quality [14]. Like the present study, the study of Hossein Rashidi et al. [15] was performed in the Iranian population, but individuals with COVID-19 were excluded from the study, and individuals without symptoms were not tested for COVID-19 infection. They found that more ART cycles were canceled at the beginning of the COVID-19 outbreak [15]. Banker et al. [16] compared in vitro fertilization (IVF) cycles at and before the beginning of the COVID-19 outbreak in India, with a study design similar to that of Hossein Rashidi et al. [15], which excluded patients with a prior positive COVID-19 test (within 90 days). Banker et al. [16] also did not find significant differences in clinical and embryological outcomes before and after the regional COVID-19 outbreak.
Although the total number of oocytes and the number of M II oocytes were lower in the present study’s exposed group, the finding was not statistically significant. Also, after applying the regression model, we found that an increase in AMH level was associated with an increased number of total oocytes, and the slightly lower number of total retrieved oocytes in the exposed group may be related to the higher AMH level in these patients. In a retrospective cohort study by Kabalkin et al. [17], the IVF cycle performance of women who had had a COVID-19 infection within the past 3 months was evaluated, and the participants’ IVF outcomes were compared to their own cycles pre-infection. The study found that the sex hormone levels, number of oocytes retrieved, fertilization rates, number of embryos created, and number of high-quality embryos were comparable before and after exposure [17]. In exploring the possibility that COVID-19 infection may affect IVF outcomes in a time-dependent manner, a retrospective cohort study showed that participants’ recent COVID-19 status had no adverse effect on fresh ART outcomes. Still, infection seemed to negatively affect oocyte yield long-term (oocyte retrieval at more than 180 days post-infection) [18]. Another study in China that investigated the impact of asymptomatic or mild COVID-19 infection found no significant differences between the COVID-19 positive group and control, except for a slight decrease in the positive group’s blastocyst formation rate [19].
One important finding of the present study is that the spontaneous abortion rate was approximately four times higher in the exposed group than in the non-exposed group (43% vs. 11%). However, the difference was not statistically significant, which may be due to the relatively small number of participants in each group. SARS-CoV-2 encodes proteins that can increase the expression of proinflammatory cytokines [20,21]. Because a higher level of proinflammatory cytokine expression is reported in the endometrial tissue of women with a history of recurrent spontaneous abortion [22], it seems possible that inflammation associated with SARS-CoV-2 could lead to spontaneous abortion.
So far, reports have been inconsistent regarding the effects of COVID-19 infection on male reproduction. Some studies showed that COVID-19 infection was correlated with significant negative effects on spermatogenesis and semen parameters [23]; however, other studies showed no significant change in sperm parameters and semen quality [24-26]. Thus, considering the lack of consensus in previous studies, we excluded couples whose husbands had experienced a recent COVID-19 infection to avoid heterogeneity between participants where possible. Additionally, though our study did not find any differences between the two groups in terms of semen parameters, we cannot be sure about the homogeneity of the two groups in terms of sperm quality for two reasons. First, we excluded only men with a history of a recent COVID-19 infection, and some men may have had an earlier history of infection; second, we assessed the status of husbands’ COVID-19 infections according to self-report, which may have caused bias.
Another finding of the present study was that after the pandemic had begun, a significantly larger proportion of participants had primary infertility (with no previous successful pregnancies), whereas before the pandemic began, the numbers of participants with primary and secondary infertility were almost the same. This may be because couples who already had at least one child had less desire to seek infertility treatment during the pandemic, since they would have to face many problems and risks caused by quarantine, financial and economic problems, or fear of the transmission of COVID-19 infection during frequent referrals to hospital, which are unavoidable during a routine infertility treatment program.
One of the strengths of the present study is that, unlike most previous studies that compared the ART outcomes of infected patients to a non-infected concurrent control group selected by their positive or negative COVID-19 test results, we selected the non-exposed group retrospectively from the patients who underwent ART in our center before the COVID-19 outbreak to ensure that they had never been exposed to SARS-CoV-2, which addresses a concern about the high probability of false positive serology in PCR tests as well as asymptomatic carriers. To control the probable effects of COVID-19 infection on sperm quality and semen parameters, we also excluded couples whose husbands had had recent COVID-19 infections. The small sample size and the lack of information about the exposed group’s pre-infection levels of sexual hormones were the main limitations of the present study. We recommend that future studies compare reproductive performance and ART/ET outcomes at different time intervals post-infection to distinguish the short and long-term effects of COVID-19 infection. We also recommend that future studies evaluate the effect of vaccination against COVID-19 to distinguish probable detrimental effects of COVID-19 infection and vaccination on reproductive function and ART outcomes, since some debates persist regarding the effects of COVID-19 vaccines on female reproductive performance and menstrual cycle regularity [27]. An additional limitation of our study is the short follow-up duration, which allowed us to report only ongoing pregnancy rates. Investigating the effects of COVID-19 infection on the maternal and neonatal outcomes of pregnancies following ART treatments is recommended for future studies.
In conclusion, this study showed that recent mild to moderate COVID-19 infection seems to have no detrimental effects on female reproductive performance and ET outcomes, from a statistical point of view. However, considering the small sample size of the present study, the statistically insignificant differences in the exposed group of fewer embryos, fewer retrieved oocytes, a lower ongoing pregnancy rate, and a higher spontaneous abortion rate might be important from a clinical point of view. Further studies with larger sample sizes are needed.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Author contributions
Conceptualization: AM, NN, LK, MFM, AMH, AT, RK. Data curation: AM, NN, LK, MFM, RK. Formal analysis: AMH. Methodology: LK, AT. Writing-original draft: AM, NN, LK, MFM, AMH, AT, RK. Writing-review & editing: AM, NN, LK, MFM, AMH, AT, RK.