In vivo and in vitro sperm production: An overview of the challenges and advances in male fertility restoration
Article information
Abstract
Male infertility can be caused by genetic anomalies, endocrine disorders, inflammation, and exposure to toxic chemicals or gonadotoxic treatments. Therefore, several recent studies have concentrated on the preservation and restoration of fertility to enhance the quality of life for affected individuals. It is currently recommended to biobank the tissue extracted from testicular biopsies to provide a later source of spermatogonial stem cells (SSCs). Another successful approach has been the in vitro production of haploid male germ cells. The capacity of SSCs to transform into sperm, as in testicular tissue transplantation, SSC therapy, and in vitro or ex vivo spermatogenesis, makes them ideal candidates for in vivo fertility restoration. The transplantation of SSCs or testicular tissue to regenerate spermatogenesis and create embryos has been achieved in nonhuman mammal species. Although the outcomes of human trials have yet to be released, this method may soon be approved for clinical use in humans. Furthermore, regenerative medicine techniques that develop tissue or cells on organic or synthetic scaffolds enriched with bioactive molecules have also gained traction. All of these methods are now in different stages of experimentation and clinical trials. However, thanks to rigorous studies on the safety and effectiveness of SSC-based reproductive treatments, some of these techniques may be clinically available in upcoming decades.
Introduction
In recent decades, spermatogonial stem cell (SSC)-based approaches to overcoming infertility caused by gonadotoxic therapy have become an important topic of investigation. The increasing survival rates of childhood cancer have drawn attention to the effects of gonadotoxic treatments on future fertility [1]. Unfortunately, sperm cryopreservation is not an ideal option for prepubertal boys who have not yet started to produce sperm. However, prespermatogonia, or SSCs that are responsible for initiating spermatogenesis at puberty, exist in prepubertal testicular tissue (TT); thus, cryopreservation of TT containing SSCs can preserve their reproductive potential [2]. Several medical centers worldwide currently use this technique and offer patients the option of freezing testicular biopsies before administering gonadotoxic therapy [3]. In addition, some centers admit patients who suffer from genetic or developmental disorders associated with prepubertal germ cell loss [4]. Currently, two major experimental protocols to restore fertility are being investigated: (1) SSC or TT transplantation and (2) SSC or TT culture [5].
The hypothetical purpose of preserving TT from biopsies is to allow for tissue autotransplantation in adulthood after the disease period. Maintaining interactions between the germ cells and their supporting somatic cells enables SSCs to regain differentiation within their natural niche [6]. Subsequently, these preserved SSCs or tissue fragments can be engrafted on a three-dimensional (3D) substrate by TT engineering. These 3D-culture systems provide a suitable microenvironment for cell attachment and specific growth factors for testicular regeneration [7]. The emergence of advanced bioengineered systems has offered new hope for maintaining male fertility through the development of functional male germ cells. Although SSC-based therapies provide an opportunity to restore fertility [8], technical and ethical barriers have limited the ability to complete spermatogenesis, and more efforts are required to establish a reliable culture system for clinical use.
Although some studies have focused on in vitro spermatogenesis resulting in mature gametes, none have found a sufficiently effective technique for differentiating human SSCs into functional sperm [9]. Despite the promising results obtained in recent years, further research is required to develop a therapeutic tool that will provide prepubertal boys and men with azoospermia the chance of fertility. This brief review highlights the next steps required to transform experimental approaches into clinical practice and emphasizes the current achievements and future challenges of fertility preservation in prepubertal boys and patients with azoospermia.
TT transplantation
TT transplantation involves the implantation of TT into various body sites, such as the testis, scrotum, and ectopic tissues [10]. One potential benefit of TT transplantation is the re-introduction of SSCs into the patient’s natural extracellular matrices. After TT transplantation, spermatogenesis can be induced through the systemic regulation of hormones, nutrition, and oxygen supply. Revascularization is also promoted in the TT grafts, which in turn generates mature sperm. The successful transplantation of TT, with subsequent offspring following intracytoplasmic sperm injection, was first reported in mice by Shinohara et al. [11] and Honaramooz et al. [12] in 2002, then in rat models [13], and later in higher mammals such as pigs, monkeys, and macaques [2,14-17].
The other option for sustaining fertility is transplantation of TT into experimental animals. The SSCs differentiate into sperm via the TT implanted in animal models, and then those cells are returned to the patient. However, no authentic cases of completed spermatogenesis using immature human TT xenografts have been reported [18]. This procedure is not yet authorized in clinical settings due to the substantial risk that germ cells can be contaminated by unidentified host tissue viruses such as retroviruses, as well as the endocrine differences between donor and recipient [12,19,20].
To date, numerous attempts to induce the maturation of human TT in vivo have been associated with only limited proliferation of SSCs. After the transplantation of TT, hypoxia and ischemic stress lead to tissue necrosis or activation of the apoptotic pathway [21], and ischemia-reperfusion may damage the SSCs’ niche as a result. Recent studies have succeeded in revascularizing testicular grafts by encapsulating the tissue or by applying molecular supplements such as angiogenic agents and antioxidants. These functionalized grafts have shown better outcomes [22].
Overall, the TT grafting technique has led to successful spermatogenesis in a range of animal models, but is still not an efficient clinical practice model because of the possibility of cancer cells spreading. Therefore, research aimed at improving the efficacy of tissue transplantation is still ongoing, and future studies must consider the significant variables affecting the survival rate of transplanted TT.
SSC transplantation
Since they can proliferate and differentiate, SSCs can restore fertility after being injected into the rete testis and ductuli afferents. A mouse model was used to evaluate SSC autotransplantation for the first time in 1994 [23], and promising results have been reported in other species since then [24-26]. Many studies have confirmed SSC migration to recipient seminiferous tubules and the formation of small colonies in those tubules after the transplantation of human SSCs into mouse testis [27,28]. However, the differentiation of autotransplanted SSCs into sperm has not been successful in humans. In autotransplantation, there is an inherent risk of reinfecting the patient with cancer cells and reintroducing the disease [10].
Attempts have been made with cell transplantation to exclude cancer cells from the testicular cell suspension by using fluorescence-activated cell sorting, magnetic-activated cell sorting [29,30], smart nanoparticles [31-33], and microfluidic devices [34]. Despite the current advancements, however, more reliable diagnostic techniques are required. Furthermore, because there are few SSCs in the testis, sufficient quantities must be created by in vitro proliferation for a successful treatment. The two major limitations in grafting efficiency include the low rate of cell proliferation in vitro and the absence of a standardized procedure with a high success rate [35].
Furthermore, the appearance of normal spermatogenesis after transplantation does not necessarily indicate normal functionality of the SSCs. These offspring may exhibit abnormal DNA methylation and low reproduction rates [36], which are probably due to the problems and inefficiencies of the SSC transplantation technique. The blood-testis barrier (BTB) can also be another major barrier in SSC transplantation. Singh et al. [37] investigated the high levels of glial cell-derived neurotrophic factor (GDNF) produced by immature Sertoli cells that resulted in increased SSC proliferation and significantly larger colonies in immature mice testes without the BTB. investigated the high levels of glial cell-derived neurotrophic factor (GDNF) produced by immature Sertoli cells that resulted in increased SSC proliferation and significantly larger colonies in immature mice testes without the BTB.
To summarize, removing cancer cells from testicular cell suspensions using specific culture conditions for the proliferation of SSCs and addressing the safety issues related to potential cell modification in the culture are concerns that should be addressed before clinical use [25].
In-laboratory sperm production using stem cells
To take advantage of assisted reproductive technologies, an infertile person must produce at least a few functional gametes. However, germ cells are not fully available in some azoospermia people, such as those with Sertoli-cell-only syndrome. Therefore, researchers have investigated the process of multipotent/pluripotent stem cell differentiation to produce functional sperm in vitro [38,39]. These studies have shown significant potential in animal models, but differences between human and other animal germ cells have prevented their widespread use in humans [40]. Several new studies are planned or currently underway to use stem cell therapy to treat male infertility [39,41]. Previous studies have reported that embryonic stem cells (ESCs) and induced pluripotent stem cells can be differentiated into germ cells in rodents, monkeys, and humans [42-44]. In studies by Hayashi et al. [42] and Cyranoski [45], sperm-like cells generated from mouse ESCs in a step-by-step process were injected into oocytes to produce offspring. Recently, two research groups produced spermatozoon-like cells from human ESCs, which were employed to treat azoospermic males [44,46]. Irie et al. [46] and Sasaki et al. [44] differentiated human ESCs into primordial germ cells (PGCs) with a gene expression pattern similar to nascent PGCs. Dong et al. [47] differentiated mouse ESCs into male germ cells using retinoic acid and placed them in special culture conditions to induce spermatogonial cell differentiation. After 6 days, differentiation of the cells was confirmed by evaluation of the acrosin gene [47]. In 2021, nonhuman primate ESCs were differentiated into spermatid-like cells by Khampang et al. [48] for the first time. Pronucleus formation was observed after microinjection of the spermatid-like cells into rhesus macaque mature oocytes. After artificial activation, they observed embryonic divisions, from the one-cell zygote stage to expanded blastocysts [48].
Mesenchymal stem cells (MSCs) are adult stem cells with the potential to enhance the efficiency of fertility restoration methods like SSC or TT transplantation and maintain fertility [48-58]. In 2006, Nayernia et al. [59] first reported that MSCs could differentiate into germ cells and express pre-meiotic germ cell markers. Shlush et al. [49] treated MSCs with retinoic acid, GDNF, putrescine, and leukemia inhibitory factor in a cell culture in vitro study. After 3 weeks, large flat cells and small round cells showed a morphology similar to Sertoli cells and germ cells. A xenotransplantation assay showed haploid cells with a flagellum-like structure that expressed meiotic markers and markers associated with spermatid cells [49]. However, these stem cell-based investigations have yet to document the production of morphological sperm. Since studies using transplantation or offspring production in humans cannot be confirmed for obvious ethical reasons, a different approach is required to verify the potential of human SSCs. It is also worth noting that a thorough examination for chromosomal abnormalities and epigenetic changes should be made to ensure that stem-cell-derived cells have normal genomes [60,61].
In vitro maturation of TTs or SSCs
Since SSC implantation into cultured testicular fragments is difficult and demands a high level of proficiency [62], an alternative approach could be the differentiation of SSCs into sperm via cell or TT culture (Figure 1) [10].
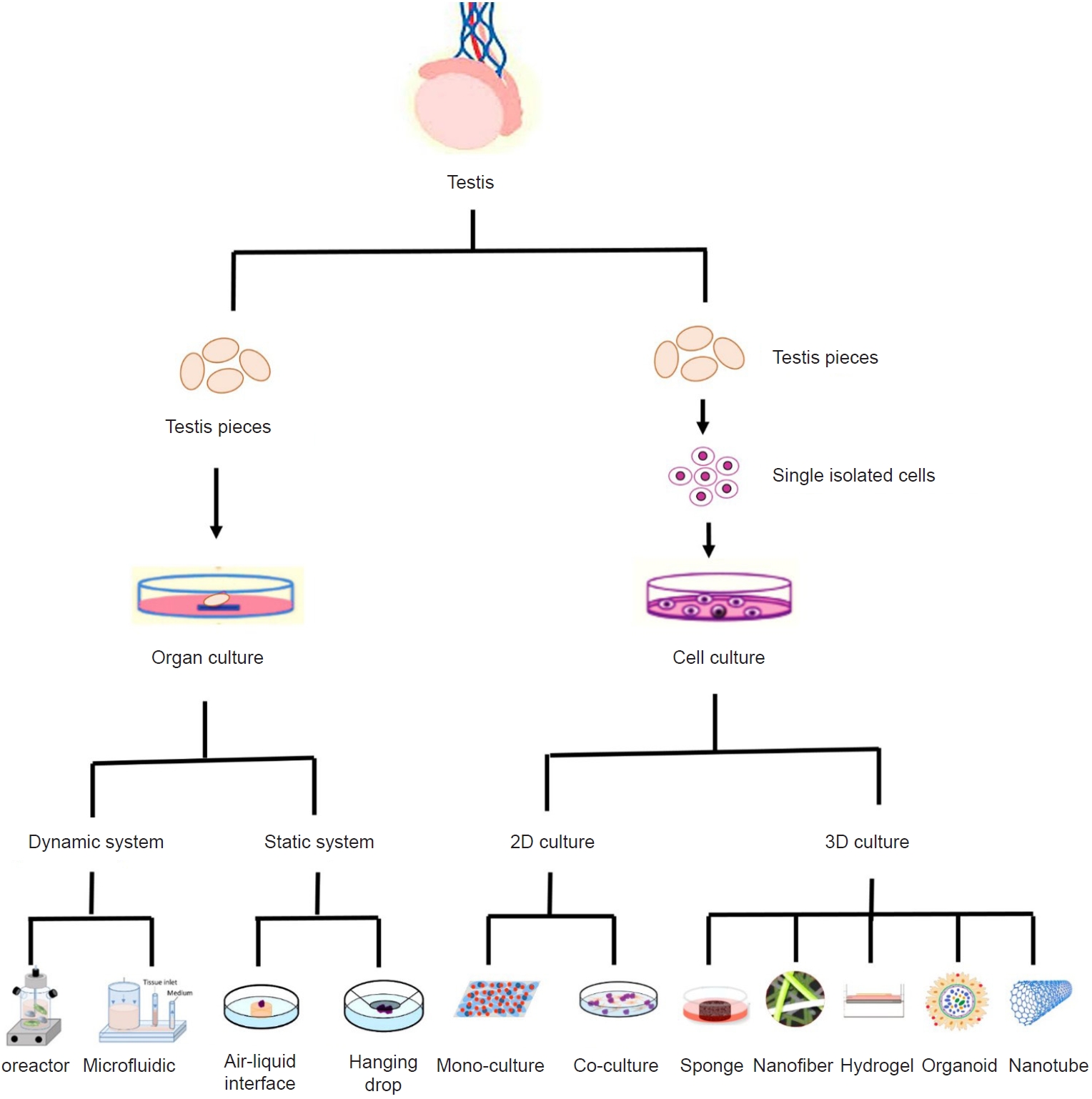
In vitro maturation of testicular tissue or spermatogonial stem cells. Testis fragments can be cultured in dynamic or static systems. In the dynamic system, tissues are cultured with a continuous flow of fresh culture medium. In the static system, the tissues are cultured at the gas-liquid interface or in a hanging drop system, which requires constant changes in the environment. Isolated testicular cells can also be cultured in two-dimensional (2D) or three-dimensional (3D) culture systems. In 2D culture systems, testicular cells are seeded on a flat 2D culture surface, with or without co-culturing with other types of cells. In a 3D culture system, cells are engrafted into a 3D environment that allows for cell-cell or paracrine interactions. The 3D cell culture systems include porous, nanofiber, hydrogel scaffold, and organoid systems.
1. TT culture
TT cultures have been used for the study of mammalian spermatogenesis because the tubules and interstitial tissue preserve their spatial integrity. The earliest laboratory-based report of spermatogenesis using rabbit TT was published in 1920; however, most of the testicular cells rapidly degenerated [63]. The first research to successfully produce functional mouse sperm in the laboratory was not documented until 2011 [64]. To restore human fertility, haploid spermatids were injected into the oocytes of patients with azoospermia in 1999 [65], which ultimately led to the birth of healthy offspring. Subsequent studies provided possible treatments for spermatogenesis disorders using TT cultures with additional supplements to cure without genetic manipulation. One such experiment was conducted by Sato et al. [66] in 2012. When stem cell factor and colony stimulating factor-1 supplements were added to immature mouse testes cultured on agarose gel, spermatogenesis increased significantly and resulted in the production of long spermatids, flagellated sperm, and live offspring after microinjection. Although supplements are a critical factor for SSC differentiation, they are insufficient on their own to generate mature human sperm in vitro. According to some studies, gonadotropins can induce SSCs to differentiate into primary spermatocytes when they are added to a culture medium containing vitamins [67,68]. Furthermore, recent studies have developed dynamic culture systems in which TT is exposed to a continuous and controlled flow of fresh culture medium [69,70]. Komeya et al. [71] reported the successful 6-month maintenance of mouse spermatogenesis using a microfluidic system. They also achieved healthy offspring following microinjection of the sperm and spermatids derived from the cultured testis [71]. In another study, testicular fragments of immature mice cultivated on agarose gel showed a lower rate of spermatogenesis than tissue produced in a perfusion mini-bioreactor, indicating that the dynamic culture system could better simulate the physiological environment of the testis [72]. Yuan et al. [73] demonstrated that self-renewing SSCs and the organization of mature seminiferous epithelium from in vitro organogenesis of the fetal gonadal ridge of human testicular in vitro-derived spermatids (from spermatogonia) could fertilize oocytes and support subsequent blastocyst formation. Although in vitro spermatogenesis using laboratory organotypic cultures preserves the 3D structure and spatial arrangement of TT, this method still faces a range of challenges. These include the need for a large volume of tissue and the ultimate loss of significant portions of that tissue, as well as the inability to genetically modify the candidate cells [74].
2. SSC culture
To overcome some of the constraints of tissue culture and minimize cell mortality caused by the scarcity of nutrition and oxygen, two-dimensional (2D) culture systems were developed for SSCs. In addition, researchers examined the addition of growth factors or the co-culturing of germ cells and feeder cells (such as Sertoli cells, Vero cells, and mouse fibroblast cells) to promote spermatogenesis [75,76]. Since 2D culture systems could not create cell-cell interactions or the exchange of nutrients and gases for stem cell differentiation, the use of 3D substrates, while maintaining normal cell morphology, was proposed [77,78].
In recent years, a wide range of synthetic polymers (synthetic carbon [79,80], polycaprolactone [81], poly-L-lactic acid [82,83], polyvinyl alcohol [84], polyamide [85], and glycolic acid [83]) and natural polymers (alginate [86,87], gelatin [88], methyl cellulose [89], collagen [90,91], fibroin [92,93], chitosan [94], Matrigel [95-97], and agarose [75,98]) have been used to fabricate scaffolds for the purpose of improving spermatogenesis. Most synthetic scaffolds were found unsuitable for SSC differentiation, whereas natural biomaterials demonstrated superior performance. In research published in 2012, mature mouse sperm were produced on a soft agar culture system (SACS) [99]. In another recent study, the completion of human spermatogenesis was observed on agarose gel plus a laminin supplement in the presence of Sertoli cells after being cultured for 74 days [100]. Analysis showed that the laminin-enhanced 3D matrix supported all physiological activities of the SSCs, including survival and proliferation, and led to the differentiation of spermatogonial cells into morphological sperm. Despite this success in spermatogenesis, the method failed to retrieve live sperm from the culture system.
In addition to the type of biomaterial, the scaffold synthesis approach could be important in the process of cell differentiation [101]. Artificial testes have been designed using various scaffolding techniques (fibrous [81-83], porous [92,102], hydrogel [89,103], and 3D printed [104-107]). Nanofibrous scaffolds could not support spermatogenesis through the final stages due to their inability to simulate the topography of TT. Over the past decade, studies have shown that extracellular matrix (ECM)-based systems of decellularized TT in the form of testicular organoids [108-116], hydrogels [116,117], sponges [102], 3D systems containing ECM [111,112], and 2D and 3D immersion culture systems [114] lead to better survival and accumulation of the SSCs for proliferation and differentiation. However, none of these studies revealed evidence of complete spermatogenesis. In our previous studies, ECM solution was used as the ioink for fabrication of a hydrogel-printed scaffold following TT decellularization with a hypertonic solution. Mouse sperm with tail-like structures that were easily separated from the surface of semi-tubular structures were identified 3 weeks after the cultivation of testicular cells [106,107]. This method can be applied to regenerate TT and restore fertility in human studies. Investigations into spermatogenesis currently focus on the secretions derived from lab-grown cell cultures, including the role of the exosomes synthesized by Sertoli cells in the survival [118,119] and differentiation of SSCs [120]. Another study also showed that epididymosomes increased the proliferation of SSCs in a decellularized TT-derived 3D system [121]. Multiple studies have reported the use of a cell-derived ECM made of a decellularized matrix to stimulate differentiation in a variety of stem cells [122-125]. Therefore, it is recommended that somatic cells from the ECM produced with decellularized TT be used to evaluate the differentiation of SSCs in the future.
Conclusions
Spermatogenic arrest and the absence of haploid male germ cells are causes of infertility in men. Since infertility secondary to cancer treatments is rising, new methods to preserve and differentiate male germ cells are needed. Researchers have offered new hope in the treatment of these patients by using the transplantation of SSCs and tissue pieces or the cell suspension-derived laboratory sperm. Sperm have been successfully produced on ECM-derived 3D printing scaffolds and SACS, which may be a step towards the creation of artificial testes.
Although fertility restoration strategies have achieved promising results in animal models, these methods are currently not suitable for the human clinical setting due to the complexity of human spermatogenesis and the lack of sufficient human tissue. In addition, more research is required to confirm that these fertility-protection strategies are safe. The simplicity of in vitro cultures and the achievements obtained thus far imply that TT transplantation can be a secure and effective treatment for fertility preservation. However, it is important to optimize this method by purifying the suspensions and removing lingering cancer cells, as well as increasing the number of SSCs in vitro before transplantation. Despite the innovations in design and fabrication technology, customization of testicular scaffolds is still a critical issue and should be further investigated to confirm its therapeutic relevance. There is reason to hope that reproductive technology will soon advance through the design of new and efficient systems that benefit humans.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Author contributions
Conceptualization: ZB, SJH. Data curation: ZB. Project administration: ZB. Visualization: ZB. Writing-original draft: ZB, MS. Writing-review & editing: SJH, MK.
