The impact of chronic insomnia disorder on menstruation and ovarian reserve in childbearing-age women: A cross-sectional study
Article information
Abstract
Objective
Diminished ovarian reserve (DOR) is a disorder characterized by impaired ovarian function. Sleep disorders are disruptions of the circadian rhythm, which appears to be closely linked to reproductive systems. This study aimed to investigate the impact of poor sleep quality on the ovarian reserve of childbearing-age women.
Methods
A cross-sectional study was conducted in China from June 2021 to March 2023. In total, 102 participants diagnosed with chronic insomnia disorder were included in the study. Questionnaires were administered to assess participants' menstrual patterns, insomnia severity, anxiety, and depression. The anti-Müllerian hormone level and the basal antral follicle count were measured for ovarian reserve evaluation. Correlation analysis and ordinal logistic regression analysis were conducted.
Results
The women with insomnia presented high percentages of hypomenorrhea, premenstrual syndrome, and dysmenorrhea (78.4%, 74.5%, and 46.1%, respectively). Severe sleep disorder in the past month was identified as an independent risk factor for hypomenorrhea and premenstrual syndrome (odds ratio [OR], 2.64 and OR, 2.688; p<0.05). The prevalence of DOR among women with insomnia (33.3%) was significantly higher than the average reported in previous studies for young women. Insomnia duration exceeding 1 year was determined to be an independent risk factor for DOR in women aged 36 to 40 years (OR, 4.5; p=0.033).
Conclusion
This study highlights the association between sleep disorders and menstrual problems. Prolonged poor sleep quality in women aged 36 to 40 years was identified as a significant risk factor for DOR. We should pay more attention to improving sleep quality in order to maintain normal ovarian function.
Introduction
The incidence of infertility has sharply increased during the past few decades. Demographic surveys indicate that millions of people worldwide are affected by infertility [1], leading many to seek out assisted reproductive technology. Couples undergoing medical treatment for infertility often experience significant emotional stress, which can profoundly disrupt their normal lives [2]. While it is known that female fertility declines with age, predicting the rate of reproductive decline in individuals is challenging. The depletion of ovarian follicles dictates the onset of reproductive senescence in women. Ovarian reserve is a key measure of a woman's reproductive potential, reflecting the capacity of follicles to grow, mature, and be fertilized. This capacity is contingent upon both the quantity and quality of the follicles and oocytes [3]. The serum concentration of anti-Müllerian hormone (AMH) and the antral follicle count (AFC), as determined by transvaginal ultrasound, are regarded as the most reliable methods for assessing ovarian reserve [4]. Diminished ovarian reserve (DOR) is a pathological condition characterized by an abnormally rapid loss of oocytes from an otherwise normal pool, leading to a reduced number and quality of oocytes. Women with DOR are more likely to face infertility [5], increased rates of miscarriage [6], adverse pregnancy outcomes in assisted reproduction treatment [7], and early menopause [8]. Although approximately 10% of young women are estimated to have DOR, the underlying causes are not well understood. Factors such as age, psychological stress, lifestyle choices, environmental influences, and iatrogenic causes have all been implicated in the development of DOR [9-12], but identifying the primary cause is complex.
Sleep health has garnered increasing attention in recent times. Roughly one-third of our lives is spent sleeping, which is an activity intimately linked with both physical and mental well-being [13]. Sleep is governed by both homeostatic and circadian processes. A typical night's sleep unfolds over four to six cycles, with each cycle lasting about 90 minutes and encompassing several stages: light slow-wave sleep, deep slow-wave sleep, and rapid eye movement (REM) sleep. The term "sleep disorder" encompasses a range of issues, including short sleep duration, poor sleep quality, and difficulty initiating sleep, all of which can disrupt the circadian rhythm. Recent research has identified a connection between sleep disorders and metabolic diseases, such as diabetes and obesity [14,15].
The circadian rhythm appears to be closely intertwined with the reproductive system. Sleep disturbances have been found to increase the risk of menstrual irregularities among female university students [16]. There is a growing body of evidence suggesting that sleep disorders are associated with impaired ovarian function, an increased likelihood of spontaneous abortion, and poorer outcomes in in vitro fertilization procedures [17-19]. Furthermore, both the biological and behavioral aspects of sleep have been observed to affect the time it takes to conceive [20]. From a biological standpoint, sleep is essential for regulating the female hypothalamic-pituitary-gonadal axis, and disruptions in circadian rhythm can influence the secretion of reproductive hormones [21]. Although some connections between sleep and female reproductive health have been established, the specific effects of sleep on ovarian reserve remain largely unexplored. To address this gap, we included female patients with chronic insomnia disorder in our study to investigate the relationship between insomnia severity and DOR, aiming to determine the role of sleep in the development of DOR.
Methods
1. Study design and participants
The study received approval from the Ethical Committee of Tongji Hospital, which is affiliated with Tongji Medical College of Huazhong University of Science and Technology (Ethics number: TJ-IRB20210613). Written informed consent was obtained from all included participants. We conducted a cross-sectional study, including female patients aged 18 to 40 years with chronic insomnia disorder who were seeking outpatient services at Tongji Hospital between June 2021 and March 2023.
2. Inclusion criteria and exclusion criteria
Our research included female participants who met all the following criteria: (1) aged between 18 and 40 years; (2) diagnosed with chronic insomnia disorder according to the International Classification of Sleep Disorders, Third Edition (ICSD-3) diagnostic criteria; (3) voluntarily signed informed consent.
To minimize the impact of organic diseases, we established the following exclusion criteria: (1) patients who were pregnant or lactating; (2) patients with primary infertility; (3) patients with hypothalamic-pituitary disease; (4) patients with a history of polycystic ovary syndrome or who have undergone ovarian surgery; (5) patients whose insomnia is attributable to concurrent physical diseases; (6) patients whose insomnia is induced by psychoactive drugs; (7) patients with sleep disorders such as REM sleep behavior disorder, periodic limb movement disorder, restless leg syndrome, or sleep-related breathing disorders; and (8) patients with severe diseases of the heart, brain, blood vessels, and connective tissue, hematologic diseases, malignant tumors, or liver and kidney dysfunction.
3. Collection of basic information
A questionnaire was designed based on the previous literature [12,22] to collect basic information such as demographic data, medical history, reproductive history, menstrual history, dietary patterns, alcohol and tobacco use, and the insomnia duration. Menstrual history encompassed the regularity of the menstrual cycle, volume of menstrual flow, length of menstrual periods, premenstrual syndrome, dysmenorrhea, and age at menarche. Insomnia duration denoted the number of years the patients reported poor sleep quality.
4. Assessment of sleep quality
All participants were required to complete two validated sleep questionnaires: the Pittsburgh Sleep Quality Index (PSQI) and the Insomnia Severity Index (ISI). These instruments assess the severity of insomnia symptoms experienced over the preceding month. The PSQI is composed of 19 items that contribute to seven component scores, which are subjective sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction [23]. Each component is scored on a scale from 0 to 3, with 0 indicating no difficulty and 3 indicating severe difficulty. The maximum PSQI score is 21, with a score greater than 5 suggesting impaired sleep quality. The ISI consists of seven questions, each rated on a 5-point Likert scale ranging from 0 to 4 [24]. The total score ranges from 0 to 28, with scores above 14 indicating poor sleep quality.
5. Evaluation of anxiety and depression
Anxiety and depression levels over the past 2 weeks were assessed in all participants using the Generalized Anxiety Disorder 7-item (GAD-7) scale and the 9-item Patient Health Questionnaire (PHQ-9). In the GAD-7, participants rated the frequency of their symptoms on a 4-point scale ranging from "not at all" to "nearly every day." The total score can vary from 0 to 21, with scores exceeding 9 suggesting the presence of severe generalized anxiety disorder [25]. The PHQ-9 is a widely utilized tool for evaluating depressive episodes [26]. Each item is scored from 0 (not at all) to 3 (nearly every day), and scores above 14 indicate moderate or severe depression.
6. Assessment of ovarian reverse
The serum AMH level and AFC in both ovaries were utilized to evaluate the ovarian reserve of the study participants. Serum AMH, which is secreted by the granulosa cells of early-stage follicles, is independent of gonadotropin influence and thus exhibits relative consistency across and within menstrual cycles. Blood samples for AMH measurement were collected on a random day of the menstrual cycle and analyzed using an AMH enzyme-linked immunosorbent assay (ELISA) kit in a consistent laboratory setting. AFC, which is the sum of 2- to 10-mm follicles in both ovaries identified during an early follicular phase transvaginal ultrasound, was consistently counted by the same experienced gynecologist between the 1st and 3rd days of the menstrual cycle. The criteria for diagnosing DOR were derived from established literature and guidelines. The threshold values for assessing ovarian reserve were established at 1.1 ng/mL for AMH [27], and 10 (bilateral ovaries) for AFC [3]. Therefore, patients with AMH <1.1 ng/mL or AFC <10 were classified as having DOR.
7. Statistical analysis
Data were analyzed using SPSS ver. 27 (IBM Corp.). The Kolmogorov-Smirnov test was utilized to assess the normality of the data. Data that were not normally distributed were represented as medians or interquartile ranges (IQR). The chi-square test was employed to compare frequencies. Due to the non-normal distribution of AMH results, the Spearman test was conducted for correlation analysis between variables and AMH. Univariate ordinal logistic regression analyses were carried out to evaluate the association between candidate variables and AMH, with odds ratios (ORs) being calculated. For categorical predictors, an OR greater than 1 suggested a poorer ovarian reserve and menstrual situation when the exposed factor was present. A p-value of less than 0.05 was deemed statistically significant.
Results
1. Characteristics of the included participants
Figure 1 shows that 126 women reported insomnia and completed our questionnaire survey. Upon evaluation, two individuals without sleep disorders (PSQI score less than 6) were excluded. Consequently, 124 women were eligible for examination of AMH and AFC. Of these, 102 women were ultimately included in the study; 55 participants underwent both examinations, while the remainder only completed the AMH test and declined the AFC test.
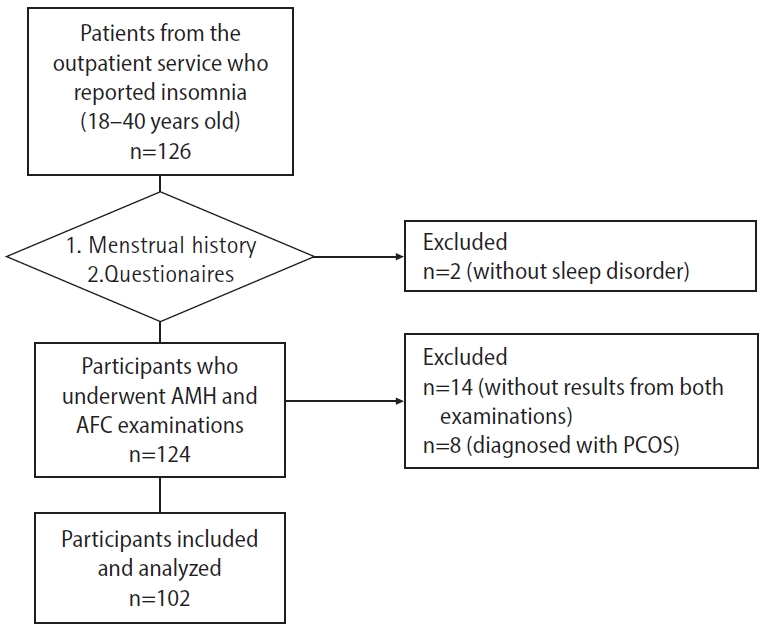
Flowchart of the participants. AMH, anti-Müllerian hormone; AFC, antral follicle count; PCOS, polycystic ovary syndrome.
The average age of the women was 33.45 years, with a range of 19 to 40 years. The majority of the women had a normal body mass index. Out of the participants, 95 women were married, representing 93.1% of the total (Table 1). None of the participants reported alcohol or tobacco addiction, nor did they report poor dietary habits.
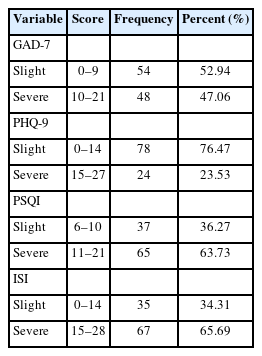
Severe insomnia was identified in 63.73% of the participants according to the PSQI results, which aligned with the findings of the ISI, demonstrating the reliability of the questionnaire survey. Furthermore, 48 individuals (47.06%) displayed significant anxiety, and 24 individuals (23.53%) suffered from severe depression (Table 2). It is well-established that sleep and mental disorders are interrelated [28]. Although limited evidence links anxiety and depression with ovarian function, we included emotional factors as candidate variables in our investigation.
2. Menstrual problems in participants
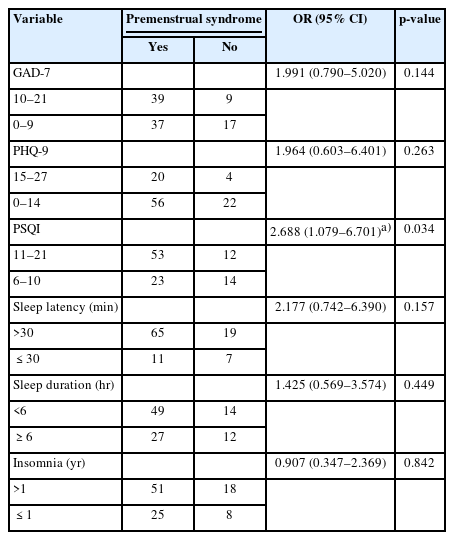
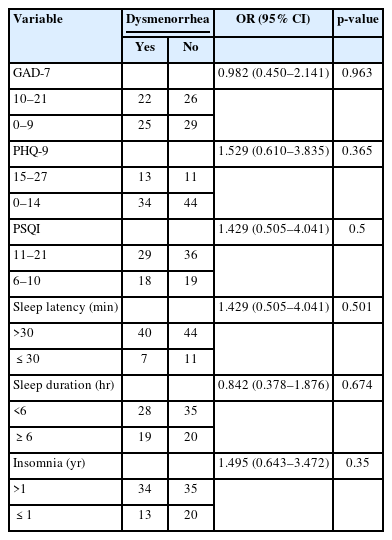
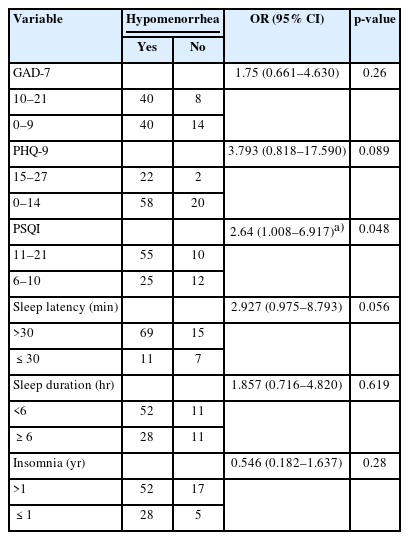
As shown in Table 3, most participants maintained a regular menstrual cycle, had a normal duration of menstrual blood flow, and reported an average age of menarche onset. However, 78.4% of the participants noted a decrease in menstrual blood flow. Seventy-six participants experienced premenstrual syndrome, while nearly half of the cohort reported suffering from dysmenorrhea. In light of the high incidence of hypomenorrhea, premenstrual syndrome, and dysmenorrhea, we performed univariable logistic regression analyses to assess risk factors, which included GAD-7, PHQ-9, PSQI scores, sleep latency, sleep duration, and duration of insomnia (Tables 4-6). The analyses indicated that severe sleep disturbances in the preceding month were significantly associated with hypomenorrhea (OR, 2.64; 95% confidence interval [CI], 1.008 to 6.917; p=0.048) and premenstrual syndrome (OR, 2.688; 95% CI, 1.079 to 6.701; p=0.034).
3. Risk factors for AMH and DOR
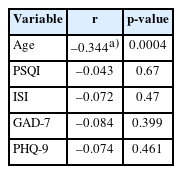
The median AMH level was found to be 2.74 ng/mL, with an IQR of 1.34 to 3.95 ng/mL. Correlation analyses were conducted between continuous variables, which included age, PSQI, ISI, GAD-7, PHQ-9, and AMH. Of these, only age demonstrated a negative correlation with AMH (r=–0.344, p=0.0004), as shown in Table 7.
Our primary focus was on the prevalence of DOR in our study participants. We identified 34 women as having DOR based on our predefined criteria, which included an AMH level below 1.1 ng/mL or an AFC less than 10. The prevalence of DOR among individuals with insomnia was 33.3%, a figure significantly higher than the average reported for young women [29]. However, after conducting univariable logistic regression analysis, we found that none of the above-mentioned risk factors were associated with DOR (Table 8). This lack of association may have been due to the limited size of our sample.

Univariable logistic regression analysis of the risk factors associated with diminished ovarian reserve
Considering age as a significant risk factor for ovarian function, we categorized the participants into three age groups: 17 individuals aged 18 to 28 years, 44 individuals aged 29 to 35 years, and 41 individuals aged 36 to 40 years (Table 1). We found that the proportion of patients with DOR was significantly higher in the 36 to 40 age group than in the 18 to 28 age group, as determined by the chi-square test (p=0.0126) (Table 9). However, there was no significant difference in the proportion of DOR between the 29–35 and 36–40 age groups (p=0.1103). We then conducted univariable logistic regression analysis to assess the risk factors associated with DOR across the different age groups (Table 10). Notably, after adjusting for age, an insomnia duration of over 1 year was identified as a significant risk factor for DOR in the 36 to 40 age group (OR, 4.5; 95% CI, 1.125 to 17.993; p=0.033). Additionally, a higher PSQI score in the 36 to 40 age group suggested a trend towards poorer ovarian reserve (p=0.069). These results indicate that the prevalence of DOR was higher in individuals with insomnia than in the general population, and that an insomnia duration exceeding 1 year specifically elevated the risk of DOR in participants aged 36 to 40 years.
Discussion
This study aimed to investigate the relationship between poor sleep quality and ovarian function in women of childbearing age. All female participants included in the study had been diagnosed with chronic insomnia disorder. The duration of insomnia over the years, sleep duration, sleep latency, and sleep scores were assessed through questionnaires. Additionally, the study examined the interconnection between sleep and mental health, with anxiety and depression disorders identified as major confounding factors.
The menstrual cycle is driven by periodic fluctuations in ovarian hormones. Prior research has indicated a high prevalence of menstrual issues among young women, with about 40% experiencing premenstrual syndrome and 32.6% reporting irregular menstrual cycles [30,31]. In our study, 78% of participants with insomnia noted a decrease in menstrual blood flow, and 74.5% suffered from premenstrual syndrome. We found that poor sleep quality was an independent risk factor for both of these menstrual issues. While our findings did not establish a link between short sleep duration (less than 6 hours) and menstrual problems, there is some evidence to suggest that insufficient sleep may disrupt the pattern of irregular menstrual cycles [32,33].
Ovarian reserve, which has a significant correlation with reproductive outcomes, was assessed in our study using AMH and AFC levels. DOR is characterized by a reduced quality and quantity of oocytes in women of reproductive age, which can lead to an increased rate of spontaneous abortions and adverse pregnancy outcomes [34]. The reported average prevalence of DOR is approximately 10%, yet in our study, 33.3% of participants were identified as having DOR. The progression of ovarian aging varies markedly among women [35]. In the absence of organic diseases, factors such as the immune system, environment, and lifestyle primarily influence the rate of ovarian aging. Our findings indicate that recent severe sleep disorders were not identified as a risk factor for DOR. However, women aged 36 to 40 years who have experienced insomnia for more than a year were found to be at a higher risk of DOR, suggesting that long-term poor sleep quality may be a key contributor to DOR.
Sleep disorders can disrupt the circadian rhythm, which in turn may affect reproductive physiology. The circadian rhythm is characterized by daily fluctuations in gene expression, metabolism, and hormone levels over a 24-hour cycle. This rhythm is governed by an internal molecular clock located in the suprachiasmatic nucleus [36]. Proper circadian regulation of reproductive hormone release from the hypothalamus, pituitary, and ovary is crucial for female fertility. Under normal sleep-wake conditions, plasma levels of hormones such as estradiol, progesterone, luteinizing hormone (LH), follicle-stimulating hormone, and melatonin display significant 24-hour rhythms [21]. Notably, clock genes modulate estrous cycle regulation, the LH surge, and the timing of fertilization [37]. These genes also affect the activity of the estrogen receptor alpha by modulating its transcriptional activity [38]. The ovary itself has an intrinsic clock, and LH has been shown to induce the expression of a multitude of genes in the ovary. This LH-induced ovarian gene expression is mediated through circadian clock proteins, including Bmal1 and Clock [37]. The silencing of Per2 and Clock genes in ovarian granulosa cells can lead to a decrease in LH receptor expression [39]. There is growing evidence that the circadian clock influences reproductive hormones, and this influence is bidirectional, as these hormones can also affect the expression of clock genes [40]. Therapeutic strategies aimed at resetting or modifying the biological clock may help to restore internal synchrony and normalize the release of reproductive hormones. While numerous studies have explored the molecular interactions between the circadian clock and reproductive function, the direct impact of sleep quality on ovarian function remains to be fully understood. Our study has shed light on the effects of long-term sleep disorders on ovarian reserve in humans, yet the precise mechanisms involved warrant further investigation.
Our study has several limitations. First, the sample size was relatively small, which may affect the generalizability of our findings. As a cross-sectional study, it cannot establish causality between sleep patterns and DOR. Therefore, larger prospective studies are necessary to further investigate this relationship. Second, our reliance on self-reported questionnaires introduces a degree of subjectivity into the evaluation of sleep quality. Future research would benefit from more objective measures, such as ambulatory polysomnography, to assess sleep quality more accurately. Third, our assessment of menstrual volume was limited to data on cycle length and subjective perceptions of bleeding amount. A more comprehensive evaluation should include the number of heavy bleeding days, the number of pads used per day and night, and other indicators such as night leaks and the presence of clots. Fourth, while we accounted for several known confounders, there may be additional unrecognized factors that could influence the results. For instance, we did not consider the causes of insomnia, such as peer pressure, work stress, or shift work, which some studies suggest may contribute to ovarian aging [41]. Fifth, we did not analyze the impact of drug therapy for insomnia, as only eight participants reported using medication for this condition. Additionally, we did not measure parameters that reflect ovarian function, such as sex hormone levels and ovarian volume.
In conclusion, our study identified an association between sleep disorders and menstrual irregularities, such as hypomenorrhea and premenstrual syndrome. Individuals with insomnia exhibited a high prevalence of DOR. Notably, long-term poor sleep quality in women aged 36 to 40 years emerged as a significant risk factor for DOR. To elucidate the relationship between sleep quality and DOR more clearly, further research with larger sample sizes is warranted.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Author contributions
Conceptualization: MG, YG, ZW, FL, HD. Data curation: MG, FL, HD. Formal analysis: MG, YG. Funding acquisition: HD. Methodology: YG, ZW. Project administration: HD. Visualization: MG, ZW. Writing-original draft: MG. Writing-review & editing: FL, HD.
Acknowledgements
We especially thank all women who participated in the study.