Impact of vitamin D supplementation as COVID-19 vaccine adjuvant on sperm parameters and sex hormones in men with idiopathic infertility: Two separate pre–post studies
Article information
Abstract
Objective
Vitamin D deficiency is a major problem for human health worldwide. The mechanisms of vitamin D in the male reproductive system are unknown. After coronavirus disease 2019 (COVID-19) vaccines were developed, doubts were raised about their possible effects on male fertility. Based on vitamin D’s function in the immune system, its potential role as an adjuvant for COVID-19 vaccines is intriguing. The aims of this study were to assess the effects of vitamin D first on sperm parameters and sex hormones, and then as an immune adjuvant on sperm parameters and sex hormones after study participants had received their second doses of COVID-19 vaccines.
Methods
Phase 1 (before the COVID-19 pandemic) included 72 men with idiopathic infertility, and phase 2 had 64 participants who received two doses of COVID-19 vaccines. Both groups were instructed to take 50,000 IU of vitamin D twice monthly for 3 months. Sperm parameters and sex hormones were assessed pre- and post-supplementation.
Results
Regular vitamin D intake for 3 months significantly increased the participants’ vitamin D levels (p=0.0001). Both phases showed a positive correlation between vitamin D intake and sperm parameters. Vaccination had no negative effects on sperm parameters and sex hormones. Vitamin D was associated with follicle-stimulating hormone (p=0.02) and testosterone (p=0.0001) in phase 2 after treatment.
Conclusion
Our results support vitamin D supplementation as an immune adjunct to COVID-19 vaccination for improving sperm parameters and hormone levels. COVID-19 vaccination is not harmful for male fertility potential, and vitamin D is an effective factor for male fertility.
Introduction
Infertility is defined as the inability to succeed in pregnancy after one year of unprotected intercourse. This condition has psychological, social, and economic effects on human life [1]. Studies indicate that 30% to 40% of infertility cases are because of the male partner’s health issues [2]. Based on World Health Organization (WHO) guidelines, some infertile men have normal sperm parameters in semen analysis. These cases are identified as idiopathic male infertility, which has no known cause. Changes in lifestyle can sometimes improve sperm parameters and restore fertility to men with idiopathic infertility [3].
Vitamin D is responsible for several biological, chemical, and physiological processes in the human body [4,5]. Essentially, vitamin D plays a primary role in regulating the balance of calcium and phosphorus [6] and is important in signaling pathways in various tissues [7,8]. It is accepted that vitamin D’s effects are more extensive in tissues that contain enzymes for metabolizing vitamin D and vitamin D receptors (VDRs) [9]. VDR expression is present in the male reproductive system, including sperm cells and testis tissue [10]. In a review paper, Cito et al. [11] reported that in animal experimental studies on mice with suppressed VDRs, several defects were seen in sperm parameters and testis histology analysis. To date, the mechanisms underlying the relationship between vitamin D and VDRs in the male reproductive system are not completely understood. Therefore, this issue warrants research.
In December 2019, a novel coronavirus spread initially from Wuhan, Hubei Province, China, and the disease it causes was named coronavirus disease 2019 (COVID-19). Though COVID-19 often affects the respiratory system directly, it also has negative effects on other tissues, including the nervous, digestive, endocrine, and urinary systems [12]. In a narrative review, we assessed the effects of COVID-19 on the male reproductive system and concluded that although COVID-19 cannot affect male fertility directly by itself, it may affect male fertility indirectly through several mechanisms. The inflammatory response after viral infection and fever are the two main biological mechanisms that affect fertility in COVID-19-infected men [13].
About 1 year after the virus’s initial spread, the first vaccine received approval from the U.S. Food and Drug Administration for emergency use to mitigate the negative effects of infections [14]. Clinical studies around the world have reported high effectiveness of vaccination against COVID-19 [15,16]. However, after the vaccine’s discovery, many doubts about its possible effects on health were raised in the non-scientific community [17]. One concern related to the potential adverse effects of COVID-19 vaccination on male fertility. In the review study mentioned above, we investigated scientific papers indicating that, in general, vaccination does not have negative effects on sperm parameters, testis tissue, or sex hormones [13].
Amid the COVID-19 pandemic, the importance of immune system support was recognized [18]. Several studies have shown that vitamin D is a key supplement for decreasing the severity of COVID-19 infection [7,19]. Biological evidence has demonstrated that vitamin D can modulate the renin-angiotensin system and the expression of angiotensin-converting enzyme 2 (ACE2) [20]. Consequently, vitamin D plays an important role in immune system function.
Based on the role of vitamin D in the immune system, the potential role of vitamin D as an adjuvant for COVID-19 vaccines is intriguing. Some papers have suggested vitamin D supplementation as an immune adjuvant to increase the efficacy of vaccination [21,22]. To date, there is no strong clinical evidence to support vitamin D's role in vaccination efficacy, and most studies published are observational and have shown varying outcomes.
Although studies have shown a direct association between serum vitamin D levels and sperm parameters, a few studies exist on the role of vitamin D in idiopathic male infertility (the aim of the first phrase of the present study). During the COVID-19 pandemic, reproductive studies focused only on antibody levels and immune protection within a limited period and did not nvestigate the factors affecting the efficacy of the COVID-19 vaccine. Therefore, the present study’s second phase aimed to address this gap.
Methods
1. Study design
The main goal for the current study’s first phase was to assess the effects of vitamin D on sperm parameters and sex hormones after 3 months. The second phase had two goals: to investigate the potential effects of vaccination on sperm parameters and sex hormones after participants had received their second dose of the COVID-19 vaccine, and to assess the effects of vitamin D as an immune adjuvant on sperm parameters and sex hormones after 3 months.
In the first phase, the influence of vitamin D supplementation on sperm parameters in idiopathic male infertility was investigated between April and November 2019. In the second phase, the effects of vitamin D supplementation as well as COVID-19 vaccination on sperm parameters in idiopathic male infertility were investigated between May and December 2021.
2. Phase 1
1) Inclusion criteria
All men with idiopathic infertility who referred to Rafsanjan urology clinic were aged 20 to 45 years and had a body mass index ≤30 kg/m2 participated in the study’s first phase.
2) Exclusion criteria
Individuals with diabetes, varicocele, cryptorchidism, drug or alcohol addiction, an age outside the study’s range, a thyroid or endocrine disorder, an immunodeficiency disorder, liver dysfunction, or systemic diseases were excluded from the study. Workers subject to chemical or metal exposure, individuals with driving-intensive jobs, and patients who had taken antioxidant supplements during the past 3 months were excluded. Individuals who did not take vitamin D supplements as instructed or who had a serum vitamin D level of ≥75 ng/mL were also excluded.
3. Phase 2
1) Inclusion criteria
All men with idiopathic infertility who referred to Rafsanjan urology clinic were aged 20 to 45 years, had a body mass index ≤30 kg/m2, and received two doses of a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine participated in the second phase. Based on the study’s vaccination plan, participants were required to have received their second dose of COVID-19 vaccine between March and August 2021, at least 1 month before the start of the study and 3 months before its end.
2) Exclusion criteria
In addition to the exclusion criteria for phase 1, recipients of a booster vaccine dose (third or subsequent dose), individuals who received two different types of vaccine doses, and patients with severe COVID-19 during the study were excluded.
4. Intervention
All participants were examined by a urologist upon their first visit, and a sperm analysis, sex hormone test, and serum vitamin D level test were requested. After the laboratory report assessment, during a second visit the patients were prescribed a 50,000 IU vitamin D pill (Daana D-Vigel 50,000 Vitamin D3; Daana Pharma Co.), to be taken once every 2 weeks for 3 months. Participants were then requested to return for a third visit with a sperm analysis, sex hormone test, and serum vitamin D level test. During the study, participants were invited to ask the researchers and the urologist any questions that they had.
5. Testing
1) Sperm analysis
Semen samples were obtained by masturbation in a sterile plastic container after 2 to 4 days of sexual abstinence. Samples were kept in an incubator at 37 °C for 30 minutes. After liquefaction, sperm parameters (count and motility) were assessed based on the 2010 WHO guidelines using a light microscope (Olympus Co.). Total sperm count was reported by count ×106, and sperm motility was classified into three categories (progressive sperm, non-progressive, and immotile) and was presented as a percentage. Sperm morphology was also measured using Papanicolaou staining tests, and normal morphology was reported as a percentage.
2) Serum vitamin D levels
Blood samples were obtained from peripheral veins for serum 25-hydroxyvitamin D (25(OH)D) testing. Serum vitamin D was measured by the enzyme immunoassay method (Immunodiagnostic Systems Ltd.). The vitamin D level was reported in nanograms per milliliter (ng/mL). We categorized the levels based on the Vitamin D Council guideline, with levels of deficient (0 to 30 ng/mL), insufficient (31 to 39 ng/mL), sufficient (40 to 80 ng/mL), and toxic (>150 ng/mL) [23].
3) Sex hormones
To measure follicle-stimulating hormone (FSH) levels, a blood sample was collected and FSH was tested using an immuno-radiometric assay (Immunodiagnostics Systems). The normal range for FSH in men is 1.5 to 12.4 IU/L [24]. Testosterone was also measured from a peripheral venous blood sample using an enzyme immunoassay (Bayer Diagnostics PLC). The normal range for testosterone in men is 2.4 to 9.5 ng/mL [24].
6. Statistical analysis
All data were input into a computer system and statistically analyzed using SPSS ver. 20 (IBM Corp.). The Kolmogorov-Smirnov test was run to assess the normal distributions of variables. In each phase, the paired sample t-test was used to assess the differences in parameters before and after the intervention for data with a normal distribution. For data that were not normally distributed, the Kruskal-Wallis test was used, followed by the Mann-Whitney U test. In each phase, the independent-sample t-test was used to compare the two post-intervention groups when the data had a normal distribution, whereas the Mann-Whitney U test was used when the data were not normally distributed. To analyze correlations, the Pearson test was used for parametric data, and the Spearman rho test was used for non-parametric data. A p≤0.05 was considered to indicate statistical significance.
7. Ethical considerations
In each phase, the study plan was explained to the participants in detail, and informed written consent was obtained. The 50,000 IU vitamin D pills were provided to the participants for free. This study was approved by the Ethical Committee of Rafsanjan University of Medical Sciences, Rafsanjan, Iran (under code IR.RUMS.REC.1397.172).
Results
In total, 200 men were diagnosed with idiopathic male infertility and screened in the urology clinic. Of these, 25 individuals were excluded from enrollment in the first phase (17 did not meet the inclusion criteria, six had vitamin D levels >75 ng/mL, and two did not agree to participate), and 32 individuals were excluded from phase 2 (11 did not meet the inclusion criteria, nine had vitamin D levels >75 ng/mL, 10 had received two different types of COVID-19 vaccines, and two did not agree to participate). Thus, phase 1 had 75 participants, and phase 2 had 68 participants. At the 3-month follow-up, three additional individuals were excluded from phase 1 (in one case, pregnancy occurred by in vitro fertilization, and two individuals did not take the required vitamin D supplements); four additional individuals were excluded from phase 2 (two individuals experienced a COVID-19 infection during the study period, and two individuals did not take the required supplements). Data from the remaining 72 participants of phase 1 and 64 participants of phase 2 were analyzed (Figure 1).

Flow diagram of participants in both study phases. COVID, coronavirus disease; IVF, in vitro fertilization.
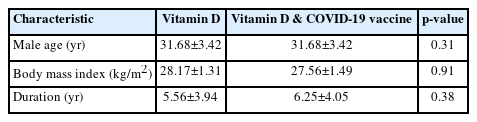
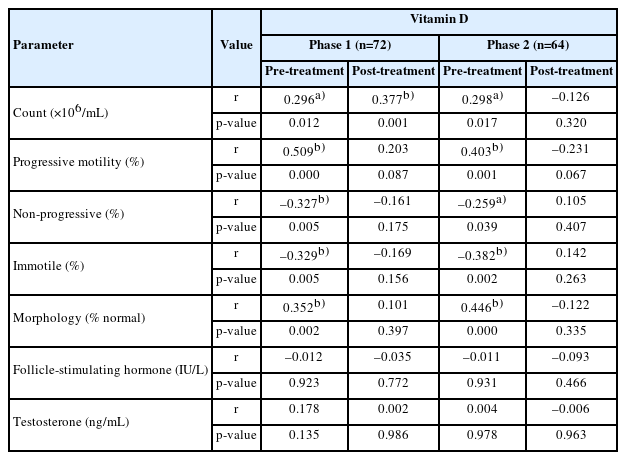
The mean ages were 31.68±3.42 and 31.68±3.42 years in phases 1 and 2, respectively (Table 1). Table 2 shows the characteristics of sperm parameters and sex hormones in each phase, pre- and post-treatment. The baseline mean vitamin D level was 29.43±8.21 ng/mL in phase 1 and 33.20±7.54 ng/mL in phase 2. A positive association was observed in both phases between vitamin D intake and sperm parameters, pre- to post-treatment. Vitamin D status did not significantly affect hormone levels in phase 1 (FSH, p=0.676; testosterone, p=0.080), but interestingly, the effects were statistically significant in phase 2 (FSH, p=0.02; testosterone, p=0.0001) (Table 2).
Before treatment, vitamin D deficiency, insufficiency, and sufficiency levels were detected in 41, 16, and 15 participants in phase 1, respectively, and in 21, 31, and 12 participants in phase 2, respectively. Supplementation elevated participants’ vitamin D levels statistically significantly in both phases (p=0.001). After treatment, no vitamin D deficiency was observed in participants (63 had sufficient levels and one had insufficient levels). No significant correlation was found between vitamin D levels and either sperm parameters or sex hormones (Figures 2 and 3).

Comparison of study parameters at different vitamin D levels in (A) pre-treatment and (B) post-treatment phases 1. SD, standard deviation; FSH, follicle-stimulating hormone.
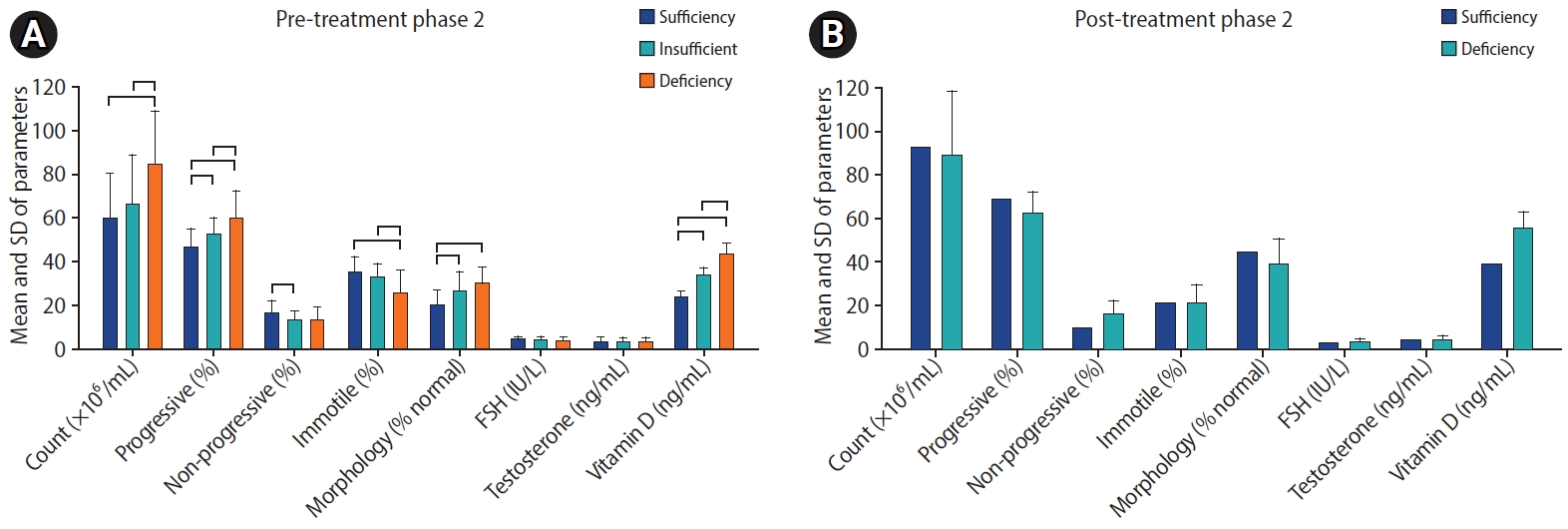
Comparison of study parameters at different vitamin D levels in (A) pre-treatment and (B) post-treatment phases 2. SD, standard deviation; FSH, follicle-stimulating hormone
Table 3 shows the correlation in each phase between sperm parameters and vitamin D . Vitamin D levels showed positive correlations with sperm count (r=0.296, p<0.01), progressive motility (r=0.509, p<0.0001), and percentage of normal morphology (r=0.352, p<0.002) in phase 1 pre-treatment. A negative correlation was found between vitamin D and non-progressive sperm motility (r=–0.327, p<0.005) as well as immotile sperm (r=0.329, p<0.005).
Discussion
Vitamin D deficiency is a global problem [25]. In 2015, Tak et al. [26] reported that 12.5% to 48.5% of men had vitamin D deficiency. Similarly, the results of the present study showed that at the start of the first phase, 54.9% and 22.22% of the participants had deficient and insufficient levels of vitamin D, respectively. At the start of phase 2, 32.8% and 48.43% of the participants had deficient and insufficient levels, respectively. We also found that vitamin D levels were significantly diminished in men with idiopathic infertility who had decreased sperm motility and morphology. The results of studies by Blomberg Jensen et al. [27] in 2016 and Rehman et al. [28] in 2018 were similar to our findings. However, a few studies have reported that vitamin D had no effects on sperm parameters [29,30]. Case selection, skin color [31], age, and geographical location [32] are the main factors identified as related to serum vitamin D levels in the literature. It is possible that differences in these factors may explain discrepancies in studies’ results, although the reasons for these inconsistent findings are not clear, and the discordant findings in the literature may be influenced by study design, sample size, participant characteristics, measurement methods, and other confounding factors.
A comparison of pre- and post-treatment measurements in phase 1 showed that vitamin D levels played a major role in improving sperm parameters. In phase 1, vitamin D levels increased significantly after treatment, and all sperm parameters (count, motility, and morphology) improved to a statistically significant extent. In a similar pre- and post-treatment study, Alzoubi et al. [33] investigated the effects of 50,000 IU vitamin D on 117 men with idiopathic infertility and demonstrated that vitamin D had favorable effects on sperm parameters, especially sperm motility, after 2 months.
Previous studies have suggested a potential link between low vitamin D levels and abnormal sperm morphology. For example, a study published in 2016 reported that men with vitamin D deficiency had a higher prevalence of abnormal sperm morphology than those with sufficient vitamin D levels [28]. Another study, published in 2017, found an association between lower vitamin D levels and increased sperm DNA fragmentation, which could be related to sperm morphology abnormalities [34]. However, other studies have not found a significant association between vitamin D levels and sperm morphology. For instance, a study published in 2015 examined the relationship between vitamin D levels and semen parameters, including sperm morphology, in a large cohort of men, but did not find a significant correlation [26]. It is important to consider that the available research in this area has certain limitations, such as variations in study design, participant characteristics, and measurement methods for assessing vitamin D levels and sperm parameters. Additionally, the mechanisms underlying the potential effects of vitamin D on sperm quality, including motility and morphology, are not fully understood.
Studies have confirmed that an increase in intracellular calcium levels leads to a higher serum vitamin D level [6]. Calcium is a key factor for sperm motility [35]. Vitamin D also plays an important role in the sperm acrosome reaction [36]. Thus, a positive relationship exists between vitamin D level and sperm maturity and quality. Previous studies have identified VDRs in the male reproductive system, specifically in Sertoli cells, Leydig spermatogenesis germ lines, and mature sperm [10], and some vitamin D metabolizing enzymes have been found in sperm heads [37]. The enzymes involved in vitamin D metabolism include 1α-hydroxylase (CYP27B1), which converts inactive vitamin D (25(OH)D) to its active form (1,25-dihydroxyvitamin D), and 24-hydroxylase (CYP24A1), which facilitates the degradation of active vitamin D. The presence of these enzymes in various cells within the male reproductive system indicates that these cells have the potential to synthesize and degrade vitamin D locally, independent of systemic vitamin D metabolism. This local metabolism of vitamin D suggests that the male reproductive organs may have a unique ability to regulate their own vitamin D status and response [38,39]. Based on these findings, we consider the effects of vitamin D on sperm parameters to be favorable. The specific roles and implications of local vitamin D metabolism in the male reproductive system are still an active area of research, and further studies are needed to fully understand the significance of this process in both animals and humans.
Researchers have recently suggested that 4-hydroxynonenal (4-HNE) may serve as an oxidative stress messenger [40]. In 2021, Shahid et al. [41] investigated the roles of vitamin D and oxidative stress in male fertility and found that 4-HNE had a negative relationship with vitamin D levels. Because 4-HNE can mediate lipid peroxidation, it plays a role in the negative impact of oxidative stress on sperm parameters in infertile men. In the presence of a sufficient level of vitamin D, the level of 4-HNE significantly decreased [41]. Consequently, the role of vitamin D in improving sperm parameters may be because of its antioxidant properties. Although we have tried to describe the known mechanisms that pertain to vitamin D’s effects on sperm parameters, we are currently unable to propose a strict, precise mechanism for how vitamin D may influence sperm parameters in infertile men.
Our results in phase 1 showed no relationship between vitamin D levels and sex hormones after treatment. The effects of hormone levels on reproductive ability have been established [42]. Several studies have reported disturbances in FSH and testosterone levels in infertile men [42,43]. Physiological studies have also identified the role of vitamin D as an important factor altering steroidogenesis (i.e., the production of hormones) [44,45]. Nevertheless, the contradictory reports on vitamin D's effects on sperm parameters and sex hormone levels in different studies indicate a collective gap in knowledge related to the exact mechanism of vitamin D's effects on human physiology.
Abbasihormozi et al. [36] reported in 2017 that vitamin D did not have any effect on male reproductive hormones. They concluded that vitamin D may not have any effect on the hypothalamus-pituitary-gonads axis [36]. In agreement with our results, Abbasihormozi et al. [36] found a positive relationship between serum vitamin D levels and sperm motility in infertile men, but they reported no association between vitamin D and sex hormone levels. It is likely that the effects of vitamin D on sperm parameters and sex hormones are distinct. In 2016, Zhu et al. [46] observed no correlation between sex hormone levels and vitamin D levels in 186 infertile men. Interestingly, they found that a higher level of prolactin correlated with vitamin D deficiency in infertile men [46]. Prolactin receptors, like VDRs, are expressed in the testis and male reproductive system [47]. An increase in the level of prolactin may disrupt spermatogenesis and hormone production through inhibition of VDRs.
During the COVID-19 pandemic, studies have investigated different factors that could be useful for preventing or mitigating COVID-19 [48]. Several studies have reported that vitamin D and antioxidants can protect the human body against the progression of COVID-19 [19] because vitamin D elevates adaptive immune responses, can alter the expression of ACE2, and can modulate renin-angiotensin homeostasis, thereby playing an important role in COVID-19 prevention [20]. However, the potential role of vitamin D in the efficacy of different COVID-19 vaccines remains ambiguous. Based on the molecular processes of immune responses in the presence of vitamin D, we speculate that vitamin D may play a balancing role in increasing COVID-19 vaccine efficacy.
The significant and positive effects of vitamin D on the immune system motivated us to investigate the potential role of vitamin D as an immune adjuvant for COVID-19 vaccines and to establish the current study’s second phase. The results of phase 2 were remarkable. Before vitamin D supplementation, the pre-treatment analysis comparing sperm parameters and sex hormone levels at different vitamin D levels showed a significant correlation between sperm parameter improvement and vitamin D levels. At deficient and insufficient vitamin D levels, sperm parameters were significantly lower than when vitamin D levels were sufficient. These results are supported by the positive effects of vitamin D in calcium homeostasis [6], the expression of VDRs in testis tissue and sperm cells [10], the antioxidant role of vitamin D [19], and the effects on prolactin levels [46], as discussed above. Beyond this, COVID-19 vaccination did not have negative effects on sperm parameters or sex hormones. In our earlier review of the literature, we found that COVID-19 vaccines were not harmful for male reproductive potential [13]. The present study’s findings add further support.
In phase 2, a positive correlation between sperm parameters and vitamin D levels was observed after treatment. In contrast to the similar results after treatment in phase 1, in phase 2 the levels of testosterone and FSH had changed significantly compared to their pre-treatment levels. We found that the levels of vitamin D in almost all participants were sufficient after supplementation of 50,000 IU vitamin D for 3 months; only one patient showed an insufficient level of vitamin D. Since almost all participants had sufficient vitamin D levels, the lack of a significant correlation between vitamin D levels and the study parameters is acceptable.
It was suggested that vitamin D plays an important role in controlling infection and inflammation during COVID-19 infection by attenuating the risk of a cytokine storm (rapid elevation in pro-inflammatory cytokines) [49]. Peng et al. [19] in 2021 investigated the effects of vitamin D on 160 patients infected with COVID-19. They reported a direct correlation between testosterone and vitamin D levels in young men, but not in old men. They also observed significantly lower testosterone levels in hospitalized men than in healthy men [19]. Testosterone may regulate the immune response, which could explain the higher level of susceptibility to COVID-19 infection in men than in women [50]. Our results showed that vaccination and vitamin D supplementation are effective for increasing testosterone levels in men with idiopathic infertility.
Wan et al. [51] demonstrated that appropriate vitamin D intake can moderate immune system responses after COVID-19 vaccination. Small et al. [52] assessed the molecular mechanisms of vitamin D in increasing the strength of the immune system. They reported that when vitamin D levels were sufficient, it exhibited binding to VDRs, which could induce the expression of two antimicrobial peptides (cathelicidin and defensin 4A). Finally, in this vitamin D-dependent antimicrobial pathway, autophagy development and innate immunity improved [52].
Similar results regarding other vaccines’ efficacy in the presence of sufficient vitamin D can shed light on the effects of the COVID-19 vaccine and sufficient vitamin D levels. For instance, Ziegler et al. [53] reported that vitamin D sufficiency can improve the efficacy of vaccination against influenza.
We found no agreement among researchers on the effective function of vitamin D as an immune adjuvant with COVID-19 vaccination. Our results showed positive effects of vitamin D on sperm parameters and sex hormones in men with idiopathic infertility who had received two doses of COVID-19 vaccines. However, we cannot definitively state that the increase in sperm parameters and sex hormones was due to the immune adjuvant properties of vitamin D along with the vaccine. Furthermore, the effects of vitamin D supplementation in the vaccine recipients cannot be ignored. Therefore, future studies may provide new insights into the effects of vitamin D on COVID-19 vaccine efficacy.
In conclusion, vitamin D deficiency has a significant negative effect on sperm parameters. Supplementation with vitamin D (50,000 IU once every 2 weeks for 3 months) was effective in increasing vitamin D levels and improved sperm parameters. Our results also support the use of vitamin D supplements as an immune adjunct to COVID-19 vaccination.
Our study has several strengths. Based on our knowledge, no published paper to date has assessed the effects of vitamin D and vaccination on male fertility. This is the main novelty and strong point of the present study. Most of our study population had low levels of vitamin D, and supplementation was effective in improving the vitamin D level to sufficiency. We minimized the effects of seasonal changes on vitamin D levels by sampling equally in both phases in spring, summer, and autumn. Having similar sample sizes between the two phases increased the accuracy of detecting intervention effects. We attempted to describe the observed mechanisms related to possible effects of vitamin D on sperm parameters and sex hormones. The similarities in the outcomes of several parameters after both phases were helpful for validating our results. The use of one type of vaccine in all patients reduced the likelihood that different effects would be observed with different vaccines and provided more reliable results. All sperm analyses and hormonal parameters were tested in a single laboratory. The comparisons made before and after treatment and the separation of the investigation into two phases are the main strengths of this study.
In the present study, lifestyle effects such as smoking, pesticide exposure, and job type were not assessed. Sperm chromatin quality and DNA fragmentation tests were also not evaluated. Participants’ levels of calcium, iron, and phosphorus were not measured, and we likewise did not measure the levels of blood immunological factors or immunoglobulin after vaccination in phase 2.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Author contributions
Conceptualization: AN, SP. Data curation: MZ, AN. Formal analysis: SP. Funding acquisition: AN, SP. Methodology: AN, SP. Project administration: AN, SP. Visualization: MZ, AN, SP. Writing-original draft: SP. Writing-review & editing: SP.
Acknowledgements
The authors would like to thank all study participants.