Phycocyanin alleviates alcohol-induced testicular injury in male Wistar rats
Article information
Abstract
Objective
Given the noteworthy implications of alcohol consumption and its association with male infertility, there has been a notable focus on investigating natural alternatives to mitigate its adverse effects. Thus, this study was conducted to assess the potential protective effect of phycocyanin extract derived from the blue algae Arthrospira (Spirulina) platensis against ethanol-induced oxidative stress, disturbances in testicular morphology, and alterations in sperm production.
Methods
Male rats were divided into four groups (five rats each): the control group received a saline solution, the ethanol exposed group (EtOH) was subjected to intraperitoneal injections of 10 mL/kg of ethanol solution at a concentration of 38% (v/v), the phycocyanin alone treated group (P) received oral administration of phycocyanin at a dosage of 50 mg/kg, and the phycocyanin-cotreated group (PE) was given oral phycocyanin followed by ethanol injections. All treatments were administered over a period of 14 days.
Results
Our findings demonstrated that ethanol exposure induced reproductive toxicity, characterized by reduced sperm production and viability, alterations in testicular weight and morphology, increased lipid peroxidation levels, and elevated oxidative enzyme activity. In addition, the ethanol-intoxicated group showed perturbations in serum biochemical parameters. However, the simultaneous exposure to ethanol and phycocyanin exhibited a counteractive effect against ethanol toxicity.
Conclusion
The results showed that supplementation of phycocyanin prevented oxidative and testicular morphological damage-induced by ethanol and maintained normal sperm production, and viability.
Introduction
Substantial data suggest a continuous decline in worldwide male fertility over the last few decades [1,2]. The World Health Organization estimates that 9% of couples worldwide struggle with fertility issues and that male factor infertility contributes to 50% of cases [3]. Several risk factors have been linked to this issue, including genetic mutations, obesity, medications, and environmental toxins and drugs such as pesticides, smoking, and alcohol consumption [4,5].
Alcohol consumption is among the most common problems leading to fertility disturbance. It is associated with impaired semen production, poor semen quality, low testosterone levels, irregularity in reproductive organ structures, and perturbation in sexual behaviour. Previous clinical studies have reported low testicular weight and interrupted spermatogenesis in men with alcohol dependence syndrome [6].
Studies have shown that alcohol can interfere with the function of the hypothalamus-pituitary-gonadal (HPG) axis and reduce the production of gonadotropin releasing hormone (GnRH), luteinizing hormone, and follicle-stimulating hormone, resulting in the impairment of the activity of Leydig and Sertoli testicular cells, which play a crucial role in testosterone production and sperm maturation respectively [7]. Moreover, alcohol metabolism generates an excess of reactive oxygen species (ROS), thereby causing a discrepancy in the oxidative stress status of the organism [8,9]. This mechanism is considered the main way by which alcohol induces ultrastructural changes in the male reproductive system. In fact, recent animal studies showed that daily ethanol (EtOH) administration increased malondialdehyde (MDA) levels and decreased antioxidant enzyme activities in the testis tissue of male rats [10], along with decreasing sperm count, motility, and viability [11].
A wide range of treatment options for male infertility has been developed, ranging from adopting lifestyle changes such as alcohol abstinence and better nutrition habits including more vitamins and trace elements, to medical interventions such as electroejaculation and sperm retrieval techniques [12]. Moreover, many pharmacological treatments have demonstrated efficacy in optimizing sperm production and motility, especially the hormonal administration of GnRH, gonadotropins, and aromatase inhibitors. Alternative medicinal plants and food supplementation have also emerged in the pharmaceutical market as potential treatment options for infertility due to their antioxidant and scavenging properties, found essentially in their secondary metabolites such as flavonoids, proteins, and phenolic compounds [13].
Phycocyanin is an important natural phycobiliprotein and the most abundant compound found in the blue algae Arthrospira platensis, also known as Spirulina, a cyanobacterium with a long history of use in traditional diets and herbal medicine [14]. Phycocyanin accounts for almost 20% of its total protein level. It is a water-soluble blue pigment composed of a chromophore bound covalently to a protein through cysteine amino acids. It has been widely used in food and cosmetic industries as a natural dye; it is also known for its interesting anti-inflammatory, antioxidant, anti-cancer, and viral properties [15-17]. Numerous animal trials attempting to evaluate phycocyanin food supplementation against inflammation and diseases induced by oxidative stress have shown that such supplementation could decrease inflammation and apoptosis markers as well as structural alteration related to acute myocardial infarction [18]. Additionally, Penton-Rol et al. [19] reviewed phycocyanin’s protective effect against neurodegenerative disorders and discussed its role in the inhibition of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity and the activation of aryl hydrocarbon receptors.
In the present study, we aimed to investigate whether phycocyanin supplementation at a dose of 50 mg/kg would prevent EtOH-induced reproductive toxicity by promoting antioxidant defence systems in male Wistar rats.
Methods
1. Animals
Male Wistar rats weighing 160 to 230 g at the beginning of the experiment were purchased from Pasteur Institute, Tunisia and housed in separate cages under controlled conditions of temperature (25 °C) and a 12:12 light/dark cycle. All animals were provided with water and food ad libitum and were acclimatized 10 days prior to the beginning of the experiment. The animals were cared for in compliance with the Institutional Ethics Committee code of practice for the Care and Use of Animals for Scientific Purposes. The experimental protocols were approved by the Ethics Committee of Faculty of Sciences, Bizerta, Tunisia (July 10, 2021, no. 1032021).
2. Experimental design
The rats were randomly divided into four groups, five animals per group, and treated for 14 consecutive days as follows. In group 1 (control group), the rats received distilled water orally (10 mL/kg). Group 2 (EtOH), the EtOH-treated group, received distilled water orally (10 mL/kg), 1 hour before intraperitoneal EtOH injection with 10 mL/kg (38% v/v) [20]. Group 3, the phycocyanin-cotreated (PE) group, received phycocyanin extract (BioAlgues Tunisia) orally at a dose of 50 mg/kg [21,22], 1 hour before intraperitoneal EtOH injection. Lastly, group 4, the phycocyanin-treated (P) group, received only phycocyanin extract at a dose of 50 mg/kg. All rats were monitored daily for body weight during the experimental period.
3. Sperm analysis
The cauda of the epididymis was minced and homogenized in 1 mL of Earle’s medium solution with 0.2% bovine serum albumin, penicillin (100 units/mL), and streptomycin (1 μg/mL), pH 7.2. The sperm suspension was incubated for 10 minutes at a temperature of 37 °C to promote the release of sperm. The sperm count, sperm motility, and viability were determined using a hemacytometer according to the method of Besley et al. [23]. The count was repeated three times for each sample to minimize error. The ratio of live and dead spermatozoa was determined using a drop of 1% trypan blue reagent (0.2%) mixed with diluted epididymal sperm suspension. Unstained spermatozoa were considered viable and stained sperm were considered dead. Sperm viability was expressed as the percentage of stained sperm of the total sperm counted [24].
4. Oxidative stress measurement
The rats’ testes, prostate, and seminal vesicles were homogenized in Tris-buffered saline. The homogenate was centrifuged at 4 °C at 9,000 rpm for 10 minutes, and the supernatants were collected. Protein levels were estimated by the Bradford method [25]. Superoxide dismutase (SOD) and catalase (CAT) activities were measured in tissue homogenates according to Misra and Fridovich [26] and Aebi [27], respectively. Lipid peroxidation was assessed by measuring the MDA level according to the Draper and Hadley [28] method. Hydrogen peroxide (H2O2) level was assessed according to Jabri et al. [29].
5. Histological analyses
The organs were fixed overnight in a 10% paraformaldehyde at room temperature. The fixed samples were dehydrated with EtOH (50% to 100%), cleared by toluene series, and embedded overnight in liquid paraffin. Sections of 5 μm were mounted on gelatine-coated glass slides cut and stained with haematoxylin and eosin solution for histopathological analyses under light microscopy.
6. Statistical analyses
First, the structure of the data was tested to assess normality (Shapiro-Wilk test) and equality of variance (Bartlett test). One-way analysis of variance (ANOVA) was performed to test for overall differences between groups, and the Tukey multiple comparisons test was used in pairwise comparisons of the treated and control groups. Data are expressed as the mean±standard error. A value of p<0.05 was considered statistically significant. Data were analysed using GraphPad Prism ver. 5 software (GraphPad Software Inc.).
Results
1. Growth rate and relative weights of organs
The EtOH group had a significantly lower growth rate than the control group. However, the PE group had no significant difference in growth rate compared to the control group (Table 1). The testis weight in the EtOH group was significantly lower than the weights in the control and P groups. Meanwhile, the PE group maintained its testis weight when compared to the control group, but showed a significant increase versus the EtOH group. Rats exposed to EtOH alone had a significantly lower prostate weight than those in the control group. However, the combination of phycocyanin with alcohol led to a recovery of the gonad relative weight. No significant differences were noticed in the seminal vesicles’ relative weights between the control group and any treated group (Table 1).
2. Sperm analysis
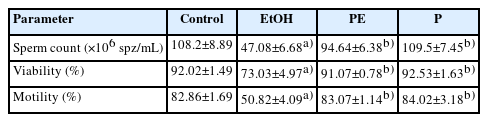
When compared to the control and P groups, the administration of EtOH alone caused a substantial decrease in sperm count as well as in the percentage of motile and viable sperm, while cotreatment with EtOH and phycocyanin was effective in maintaining sperm parameters. No significant changes were observed in sperm parameters between the P group and the control group (Table 2).
3. Lipid peroxidation and hydrogen peroxide levels
Lipid peroxidation was evaluated by examining MDA levels, which were significantly higher in the EtOH rats’ testes, prostate, and seminal vesicles than in the control and P groups. Interestingly, co-administration of EtOH and phycocyanin significantly lowered the MDA level when compared to the EtOH group. However, exposure to phycocyanin alone had no significant effect on this parameter (Figure 1).

Malondialdehyde (MDA) levels in the reproductive tissues of the control and treated groups: (A) testes, (B) prostate, (C) seminal vesicles. Values are represented as mean±standard error. EtOH, ethanol-treated group; PE, phycocyanin-cotreated group at 50 mg/kg; P, phycocyanin-treated group at 50 mg/kg. a)p<0.05 as compared to the control group; b)p<0.05 as compared to the EtOH group.
Moreover, H2O2 production in the testes, prostate, and seminal vesicles of the EtOH group was significantly higher than in the control and P groups. In contrast, the co-administration of phycocyanin resulted in significantly lower H2O2 levels in all reproductive organs for the PE group than in the EtOH group (Figure 2).

H2O2 levels in reproductive tissues of the control and treated groups: (A) testes, (B) prostate, (C) seminal vesicles. Values are represented as mean±standard error. EtOH, ethanol-treated group; PE, phycocyanin-cotreated group at 50 mg/kg; P, phycocyanin-treated group at 50 mg/kg. a)p<0.05 as compared to the control group; b)p<0.05 as compared to the EtOH group.
4. Oxidative stress enzyme activities
Testicular SOD activity was significantly higher after subacute EtOH exposure (EtOH group) than in the control and P groups. Conversely, co-administration of EtOH and phycocyanin balanced the depletion of this antioxidant enzyme, whereas exposure to phycocyanin alone had no significant effect as compared to the control group.
Unexpectedly, the rats exposed only to EtOH showed significantly higher SOD activity in their prostates and seminal vesicles than the rats in the control and P groups. However, co-administration of phycocyanin with alcohol maintained SOD status in these accessory genital glands (Figure 3).

Superoxide dismutase (SOD) activity in the (A) testes, (B) prostate, and (C) seminal vesicles of the control and treated groups. Values are represented as mean±standard error. EtOH, ethanol-treated group; PE, phycocyanin-cotreated group at 50 mg/kg; P, phycocyanin-treated group at 50 mg/kg. a)p<0.05 as compared to the control group; b)p<0.05 as compared to the EtOH group.
Figure 4 shows that in contrast to the control and P groups, EtOH exposure in the EtOH group also induced significantly higher CAT activity in the testis, prostate, and seminal vesicle tissues. Interestingly, those changes were balanced by phycocyanin co-administration. No significant changes were observed among the control, PE, and P groups.
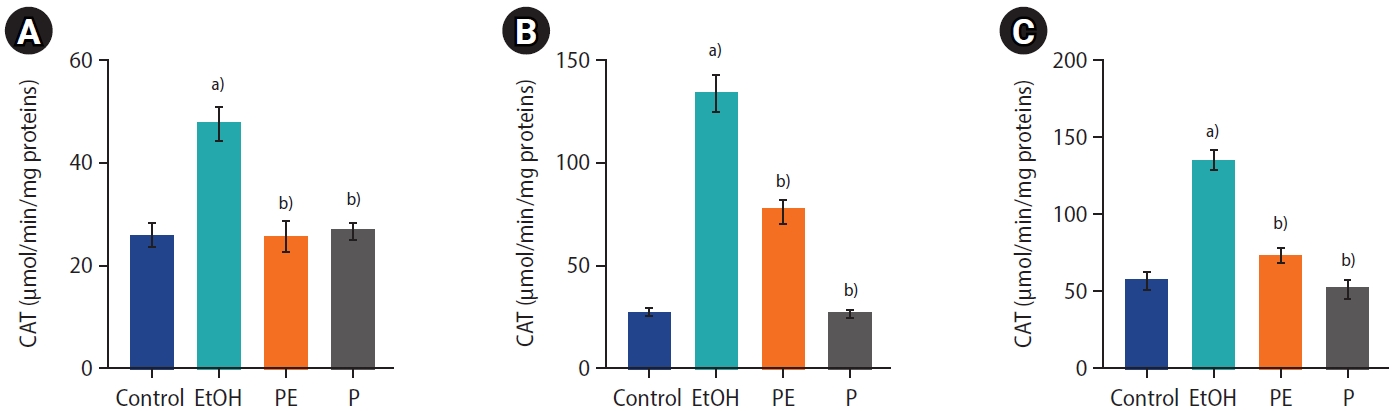
Catalase (CAT) activity in the (A) testes, (B) prostate, and (C) seminal vesicles of the control and treated groups. Values are represented as mean±standard error. EtOH, ethanol-treated group; PE, phycocyanin-cotreated group at 50 mg/kg; P, phycocyanin-treated group at 50 mg/kg. a)p<0.05 as compared to the control group; b)p<0.05 as compared to the EtOH group.
5. Testis histology
The testicular histology of the control and P groups displayed a typical seminiferous tubule structure, characterized by the presence of various components involved in the process of spermatogenesis (Figure 5A, 5B). The luminal space within the seminiferous tubules exhibited normal spermatozoa content, and the basal region displayed clear individualization (Figure 5C).
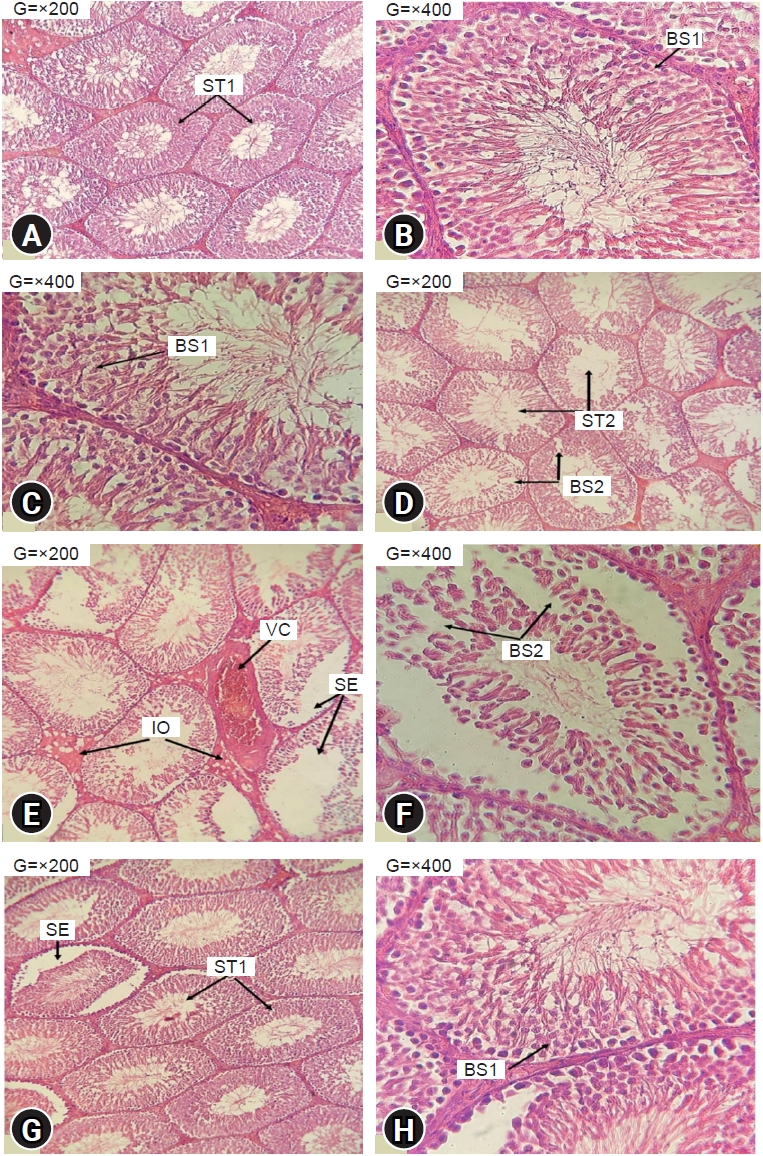
Effects of ethanol and phycocyanin on testis histology. Photomicrography of haematoxylin and eosin-stained sections of control rats (A, B); testes from rats exposed to ethanol 38% (D, E, F); testes from rats cotreated with ethanol and phycocyanin at 50 mg/kg (G, H); testes from rats exposed to phycocyanin alone at 50 mg/kg (C). ST1, normal aspect of seminiferous tubules fully loaded with spermatozoa; ST2, seminiferous tubules poorly loaded with spermatozoa; SE, sloughing of the epithelium; BS1, normal aspect of basal seat; BS2, basal seat with vacuolised appearance; VC, vascular congestion; IO, interstitial oedema.
In contrast, the histological examination of EtOH testes demonstrated significant alterations. These included the atrophy of the seminiferous tubules, with fewer spermatozoa within the lumen, sloughing of the epithelium, and disruption of the spermatogenesis process. Additionally, vascular congestion and interstitial oedema were evident (Figure 5D, 5E), and the basal layer displayed a vacuolated appearance (Figure 5F).
Remarkably, the PE rats experienced a protective histological feature in their testes. The basal layer of the seminiferous tubules remained well individualized, in addition to a fully loaded lumen (Figure 5G). Nevertheless, certain seminiferous tubules demonstrated a sloughing of the epithelial area (Figure 5H).
Discussion
Excessive alcohol consumption is a common issue among many societies, and it is reported to negatively affect human health and to have short- and long-term impacts, namely behavioural alterations, cardiovascular complications, liver diseases, and several types of cancer [30]. Several studies have demonstrated that EtOH consumption results as well in poor male reproductive capacity, including altered sexual behaviour [31], low sperm quality, and testosterone production [32]. The purpose of the present study was to investigate the protective effects of phycocyanin extract from A. platensis on reproductive parameters in adult male rats. Our results showed that the exposure of rats to 38% v/v EtOH for 14 consecutive days significantly lowered their growth rate as compared to the control group. Our result agrees with previous data [33]. The decrease in growth rate might be due to the mechanism by which alcohol depresses intestinal function and promotes a false feeling of satiation, thereby reducing food intake [34]. Remarkably, rats subjected to co-exposure of EtOH and phycocyanin, and those exposed solely to phycocyanin, exhibited consistent and unimpaired growth rates. One explanation for the protective effect of phycocyanin against weight loss is its ability to enhance the functional activities of the gastrointestinal tract by improving nutrient utilization and absorption. Additionally, phycocyanin has been implicated in the modulation of gut microbiota, promoting the proliferation of beneficial bacterial phyla such as Bifidobacterium and Lactobacillus, while simultaneously reducing the abundance of harmful bacteria like Enterococcus and Escherichia coli. This modulation of the gut microbiota can influence intestinal function and permeability, as well as foster a healthier gut environment [35]. Furthermore, studies emphasize the importance of optimal protein intake in preserving muscle mass and promoting an overall healthy growth rate [36]. Our findings align with the previous study of Husain et al. [37], demonstrating that phycocyanin supplementation at 200 mg/kg effectively counteracted the weight loss induced by streptozotocin in male rats.
The structure and function of male reproductive organs are intricately associated with androgen levels and the proper functioning of Leydig and Sertoli cells, which play vital roles in the spermatogenesis process. Consequently, any disruption in the hormonal pathway can lead to abnormalities in the reproductive organs. Therefore, assessing organ weight can serve as a crucial indicator of the effects of alcohol on the reproductive system.
In line with this, our study examined the impact of EtOH with a concentration of 38% v/v on testis and prostate size. Remarkably, a significant reduction in testis index was observed in the EtOH group compared to both the control and P groups. Previous research has already demonstrated the potential influence of alcohol on testosterone production, including the stimulation of beta-endorphin release, disturbances in corticosterone levels, and activation of the aromatization process, which converts testosterone into oestrogen. These processes are believed to contribute to testicular and accessory sex gland atrophy [38]. Interestingly, the concurrent administration of EtOH and phycocyanin demonstrated the preservation of a normal testis size in rats. Previous in vitro studies have highlighted the cytoprotective properties of phycocyanin, since it enhances cell viability and downregulates necroptotic pathways in GC-1 SPZ cell models. Phycocyanin has been observed to enhance testosterone release, stabilize feedback mechanisms, and improve spermatogenesis [39]. Furthermore, it has been reported that the structure of the phycocyanin chromophore exhibits similarities to bilirubin, which has been shown to inhibit the NADPH oxidase system responsible for triggering oxidative stress and apoptotic pathways within the cell membrane of the testis [40]. In this context it is noteworthy to consider that the activation of the NADPH system is one of the pathways involved in EtOH oxicity [41]. Taken together, these collective findings underscore the beneficial effects of phycocyanin in conferring protection to the testes and its potential to support the healthy development and function of male reproductive organs.
Sperm analysis is a common measurement to indicate the state of testicular function, and male fertility, a low sperm count, and poor sperm quality are associated with reduced fertility [42]. Our results showed that the EtOH rats had a smaller number of spermatozoa as well as a lower percentage of sperm viability and motility when compared with the control group. The alteration of sperm count and quality may be explained by the direct and indirect effects of EtOH on reproductive organs and hormones. It is suggested that EtOH decreases testosterone production by interfering with HPG axis activity and inhibits the expression of testicular proteins, namely androgen receptor proteins, heat shock protein (HSP70), and tyrosine-phosphorylated protein, which are associated with androgen binding, apoptosis prevention, and progressive sperm motility [43]. Furthermore, a recent study that investigated the effects of alcohol on testosterone synthesis in men has shown that acute consumption of a low or moderate dose of alcohol increases serum testosterone levels, while excessive or chronic absorption reduces androgen production with an increase in HPG axis activity, inflammation, and oxidative stress [44]. It has also been reported that alcohol dehydrogenase is present in the interstitial cells and seminiferous tubules of the testes, which promotes cell damage during alcohol metabolism by enhancing toxic radical production and, therefore, inducing histopathological alterations [45].
Our study yielded intriguing results, demonstrating that the administration of phycocyanin, either alone or in combination with EtOH, had positive effects on sperm production, viability, and motility. The membranes of spermatozoa contain substantial quantities of polyunsaturated fatty acids, making them highly susceptible to damage induced by ROS, oxidative stress, and subsequent apoptotic effects. These factors lead to a decline in seminal parameters, which aligns with the aforementioned deleterious effects associated with alcohol consumption [46]. In this regard, various in vitro and in vivo investigations have demonstrated that phycocyanin exhibits ROS scavenging and anti-apoptotic properties. This is attributed to its ability to inhibit the cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2) pathways, as well as its capacity to reduce the expression of inducible nitric oxide synthase induced by lipopolysaccharide and modulate the cytochrome P450 pathway [17]. Moreover, a study has found that phycocyanin can maintain normal serum testosterone levels and preserve sperm quality parameters in mice subjected to cadmium intoxication. The authors propose that the protective effects of phycocyanin can be attributed to its high cellular penetration and antioxidant capabilities [47].
In the present study, EtOH exposure also resulted in significantly higher levels of lipid peroxidation and H2O2 in the testis, prostate, and seminal vesicle tissues for the EtOH group than for the control and P groups. Our results are consistent with previous studies that demonstrated that oral or intraperitoneal EtOH exposure induces the production of free radicals and lipid peroxides [48,49]. It has been reported that EtOH metabolization by cytochrome C (Cyp450) within the microsome and mitochondria leads to excessive production of hydroxyl radicals, namely O-2 and H2O2, due to the high NADPH activity of Cyp540 [50]. Moreover, it has been discussed that EtOH may enhance the activity of the enzyme xanthine oxidoreductase, which reacts consequently with acetyl aldehyde, a primary metabolic compound of EtOH metabolism, and that it may regenerate ROS such as H2O2 [51]. High level of ROS may lead to lipid damage, particularly lipid molecules with carbon double bonds, such as polyunsaturated fatty acids. ROS initiate their reaction by the abstraction of a hydrogen molecule from the carbon bonds, which results in the production of lipid radicals and a subsequent propagation of chain reactions. The oxidation of cell lipids, namely membrane phospholipids, produces several primary and secondary products, among them MDA, which is considered one of the most toxic and mutagenic products [52]. Combined administration of EtOH and phycocyanin in the current study resulted in significantly lower lipid peroxidation and H2O2 production in all reproductive organ tissues as compared to the EtOH group. This can be explained by the scavenging properties of phycocyanin; in fact, previous in vivo and in vitro studies have demonstrated that phycocyanin extracted from A. platensis reduced ROS levels [14] and inhibited lipid peroxidation in rat liver microsomes [53].
SOD and CAT are the first line of antioxidant enzymatic defence. SOD is a metalloenzyme divided into four different subgroups based on its metal factors and distributed throughout different subcellular compartments. The SOD defence mechanism against ROS consists of reducing the superoxide anion (O2-) level in a cell by converting it into H2O2 [54]. This process is completed by CAT activity, which neutralizes H2O2 and breaks it down into harmless molecules of oxygen and water [55]. In our study, exposure to EtOH induced a perturbation of the activity of antioxidant enzymes within the testis, prostate, and seminal vesicle tissues. The EtOH group showed significantly higher activity of SOD and CAT than the control and P groups. The difference in SOD and CAT activities may be a response to the high level of MDA and H2O2 production. Our data are partially consistent with a previous study of alcohol induced testicular toxicity [56].
A histological investigation was carried out for a better understanding of alcohol-induced testicular atrophy. Microscopic examination of the control and P groups showed a normal testicular structure characterized by well-developed seminiferous tubules abundantly populated with spermatozoa. Conversely, the EtOH group displayed lesions in the epithelium of the seminiferous tubules, including widened interstitial spaces and a vacuolated appearance of the basal region, indicative of disrupted spermatogenesis. Moreover, the lumens of the seminiferous tubules in the EtOH group showed fewer spermatozoa compared to the control group. Notably, vascular congestion and interstitial oedema were also observed. Our results are consistent with an earlier report following exposure to 10% EtOH [57].
Remarkably, a histological analysis of the testes from the PE rats cotreated with EtOH and phycocyanin at a dosage of 50 mg/kg demonstrated a well-preserved structure of the seminiferous tubules, characterized by a clearly individualized basal region and lumens filled with spermatozoa, as compared to the EtOH group. These findings highlight the potential protective effects of phycocyanin against EtOH-induced testicular damage.
In the present study, phycocyanin alone at the dose used did not have significant effects on the parameters investigated. No differences were observed between the P and control groups. However, when associated with EtOH, a pro-oxidant agent, phycocyanin exhibited a protective effect by relieving all alcohol-related adverse effects. This result may be explained mainly by its substantial antioxidant potential in reducing the oxidative stress and ROS levels. Indeed, the lack of significant effects of phycocyanin alone could be explained by the low level of oxidative stress in healthy rats not exposed to EtOH and by the absence of toxicity of phycocyanin consumption as reported in previous studies [58,59].
In conclusion, the findings of our study provide evidence that alcohol induces oxidative stress damage in the testes, prostate, and seminal vesicles. It also leads to disturbances in testicular histology and a decline in sperm functional parameters. However, phycocyanin cotreatment ameliorates the alcohol-induced perturbations by mitigating oxidative stress damage and preserving the spermatogenesis process. These results suggest the potential of phycocyanin as an alternative to counteract the detrimental effects of alcohol on male reproductive health.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Author contributions
Conceptualisation: MS. Data curation: OT. Formal analysis: SG. Methodology: DH. Project administration: KBR. Visualisation: TL. Wrinting original draft: OB. Writing review & editing: WK.
Acknowledgements
The authors express their sincere thanks to Professor Youssef Krichen, CEO of Bio Algae Tunisia, for the free supply of phycocyanin, and to Dr Sihem Ben Hassine for her technical assistance.