Clinical outcomes of preimplantation genetic testing for aneuploidy in high-risk patients: A retrospective cohort study
Article information
Abstract
Objective
The purpose of this study was to evaluate the impact of preimplantation genetic testing for aneuploidy (PGT-A) on clinical outcomes among high-risk patients.
Methods
This retrospective study involved 1,368 patients and the same number of cycles, including 520 cycles with PGT-A and 848 cycles without PGT-A. The study participants comprised women of advanced maternal age (AMA) and those affected by recurrent implantation failure (RIF), recurrent pregnancy loss (RPL), or severe male factor infertility (SMF).
Results
PGT-A was associated with significant improvements in the implantation rate (IR) and the ongoing pregnancy rate/live birth rate (OPR/LBR) per embryo transfer cycle in the AMA (39.3% vs. 16.2% [p<0.001] and 42.0% vs. 21.8% [p<0.001], respectively), RIF (41.7% vs. 22.0% [p<0.001] and 47.0% vs. 28.6% [p<0.001], respectively), and RPL (45.6% vs. 19.5% [p<0.001] and 49.1% vs. 24.2% [p<0.001], respectively) groups, as well as the IR in the SMF group (43.3% vs. 26.5%, p=0.011). Additionally, PGT-A was associated with lower overall incidence rates of early pregnancy loss in the AMA (16.7% vs. 34.3%, p=0.001) and RPL (16.7% vs. 50.0%, p<0.001) groups. However, the OPR/LBR per total cycle across all PGT-A groups did not significantly exceed that for the non-PGT-A groups.
Conclusion
PGT-A demonstrated beneficial effects in high-risk patients. However, our findings indicate that these benefits are more pronounced in carefully selected candidates than in the entire high-risk patient population.
Introduction
Aneuploidy, defined as an abnormal number of chromosomes, is the most common genetic abnormality in human embryos. It is responsible for the majority of failed implantations and accounts for over half of all missed abortions, miscarriages, and congenital birth defects [1]. Aneuploidy arises from chromosomal errors during meiosis I and/or II in oocytes [2,3]. However, embryonic chromosomal errors cannot be accurately detected through embryo morphological assessments, whether using basic methods or the more recently developed time-lapse morphological kinetic embryo selection [4,5]. As such, preimplantation genetic testing for aneuploidy (PGT-A) is currently the most valuable screening method for chromosomal status and aneuploidy in human embryos. Conversely, embryo biopsy is one of the most challenging laboratory procedures.
PGT-A, previously known as preimplantation genetic screening, was initially introduced in the 1990s through the use of fluorescent in situ hybridization performed on a polar body or blastomere biopsy. This method was shown to be more efficient than cytogenetic analysis in identifying aneuploidy or abnormal chromosomal errors in human embryos [6]. However, it cannot be used to evaluate all chromosomes and exhibits relatively high rates of mosaicism [7]. Consequently, new genetic testing platforms for comprehensive chromosome screening (CCS) of 24 chromosomes have been developed and implemented. These platforms include array comparative genomic hybridization (aCGH), single-nucleotide polymorphism, quantitative real-time polymerase chain reaction, and next-generation sequencing (NGS) [8]. The introduction of oligonucleotide probe arrays for CGH, along with improvements in whole genome amplification, has advanced and popularized embryo aneuploidy testing. Among the available methods, the NGS technique is the most suitable for aneuploidy testing of trophectoderm (TE) biopsies. This is due to its accurate results, high throughput, cost-effectiveness, and widespread use in laboratories. Recently, this technique has been applied to the analysis of embryonic cell-free DNA released into the culture medium during the blastocyst stage [9]. Despite its simplicity and ease compared to biopsy, noninvasive PGT-A still presents certain challenges.
Benefits of PGT-A include the preferential selection of euploid embryos for transfer along with the reduction of miscarriage and multiple gestation rates. Additionally, this approach ensures an excellent ongoing pregnancy rate/live birth rate (OPR/LBR), reduces the time to pregnancy, and facilitates elective single embryo transfer (ET). Thus, PGT-A is recommended for treating patients at an increased risk of aneuploid embryos, such as those of advanced maternal age (AMA) and those affected by recurrent implantation failure (RIF), recurrent pregnancy loss (RPL), or severe male factor infertility (SMF) [10]. The benefits of using CCS on TE cells in PGT-A have been documented in randomized controlled trials (RCTs) [11,12]. However, conflicting findings have also been reported [13,14], leaving the debate on its clinical outcomes unresolved. Therefore, a need exists for more data on the clinical outcomes of PGT-A using TE biopsy with CCS methods.
In the absence of robust contemporary data, we performed a single-center cohort study. The aim was to analyze the clinical outcomes associated with the use of TE biopsy, aCGH, or NGS with 24-chromosome screening compared with non-PGT-A during in vitro fertilization (IVF) cycles in high-risk patients.
Methods
1. Study population
This single-center retrospective cohort study was conducted using data from 1,368 patients and the same number of cycles. The participants underwent reproductive treatment either with (in 520 cycles) or without (in 848 cycles) PGT-A at our IVF center between January 2019 and July 2022. During this period, our center transitioned from using aCGH (n=143, 27.5%) to NGS (n=377, 72.5%) for PGT-A. Only patients with major indications for PGT-A, namely AMA, RIF, RPL, and SMF, were screened. AMA was defined as an age of 38 or older, whereas RIF was defined as the absence of a gestational sac on ultrasound following the transfer of at least three fresh or frozen cycles [15]. RPL was defined as two or more miscarriages before 20 weeks of pregnancy [16,17], and SMF encompassed azoospermia (both obstructive and nonobstructive) and severe oligoasthenoteratozoospermia (defined as a sperm concentration of less than 5×106/mL, motility under 40%, and less than 4% morphologically normal spermatozoa). Oocyte donations and patients with monogenic diseases or abnormal karyotypes were excluded. In the PGT-A group, only euploid embryos were transferred, and only the first frozen embryo transfer (FET) per patient was included.
In this study, the infertile patients with PGT-A cycles were distributed as follows: AMA (n=233), RIF (n=130), RPL (n=80), and SMF (n=77). The patients of the control (non-PGT-A) group were subdivided as follows: AMA (n=289), RIF (n=266), RPL (n=132), and SMF (n=161). Tables 1-4 detail the baseline demographic characteristics and parameters for each group. The Institutional Review Board (IRB) of Maria Fertility Hospital granted approval for this study (IRB reference number: 2021-005). Written informed consent by the patients was waived due to a retrospective nature of our study.
2. Ovarian stimulation, oocyte collection, and insemination
Ovarian stimulation was performed using a combination of long and short gonadotropin-releasing hormone agonists (Decapeptyl, Ferring Pharmaceuticals; or Lorelin Depot, Dongkook Pharm) and antagonists (Cetrotide, Merck-Serono; or Orgalutran, Organon), along with human menopausal gonadotropin (IVF-M HP, LG Chem; or Menopur, Ferring Pharmaceuticals). The gonadotropin dosage was individually adjusted based on the follicular response, as observed via transvaginal ultrasonography. Once the leading one or two follicles reached a mean diameter of ≥18 or ≥17 mm respectively, a subcutaneous injection of 250 µg of recombinant human chorionic gonadotropin (hCG) (Ovidrel, Merck-Serono) was administered. Oocyte retrieval was performed 35 to 36 hours after hCG injection and was followed by fertilization via IVF and/or intracytoplasmic sperm injection. Fertilization was assessed 17 to 18 hours after insemination by identifying the presence of two distinct pronuclei and two polar bodies.
3. Embryo culture and blastocyst biopsy
Embryos were cultured until they reached the blastocyst stage, and TE biopsy was performed between days 5 and 7. The biopsy process entailed securing the blastocyst in the proper position using a holding pipette, and then creating a small hole in the zona pellucida (ZP) using a ZILOS-tk laser system (Hamilton Thorn Ltd.). Following this, a 21-μm polished biopsy pipette (TPC; CooperSurgical, Inc.) was inserted into the ZP through the opening. Next, 5 to 10 TE cells were aspirated into the biopsy pipette, and these cells were then separated from the blastocyst using the recently reported new laser and flicking biopsy method [18]. The biopsied cells were subsequently rinsed four to five times, then placed in 0.2-mL polymerase chain reaction tubes containing 2.5 μL of phosphate-buffered saline. These tubes were then stored at −20 °C until further processing via aCGH or NGS. All blastocysts were cryopreserved in preparation for FET.
4. Testing for aneuploidy
Cells obtained from the biopsy were analyzed using tools from different commercial genetic testing companies according to the technique employed (aCGH, MGmed; NGS, GenoBro or Igenomix Korea). First, complete genome amplification was performed; the biopsied cells were then analyzed via aCGH using Illumina 24sure+ arrays (Illumina Inc.) or through NGS using a synthesis sequencer (Thermo Fisher Scientific).
5. Blastocyst vitrification and FET
Vitrification, thawing of biopsied blastocysts, endometrial preparation, and transfer procedures were performed as previously described [19]. All embryos were transferred in either natural or hormonally prepared cycles, according to the Korean Ministry of Health and Welfare guidelines. If more than one euploid embryo was available, one or two of the highest-quality euploid embryos were transferred. However, if only a single euploid embryo was available, it was transferred irrespective of its quality.
6. Clinical outcome measures
The clinical outcomes evaluated in this study included clinical pregnancy rate (CPR), implantation rate (IR), OPR/LBR, chemical pregnancy loss, and early pregnancy loss. CPR was defined as a serum quantitative hCG level exceeding 100 mIU/mL, coupled with the presence of a gestational sac as observed on a transvaginal ultrasound at 6 to 7 weeks of gestation. IR was determined by dividing the number of gestational sacs observed on ultrasound by the number of embryos transferred. OPR/LBR referred to the birth of a neonate at or beyond 20 weeks of gestation. Chemical pregnancy loss was characterized by a serum quantitative hCG level above 100 mIU/mL, but without the presence of a gestational sac on transvaginal ultrasound. Finally, early pregnancy loss was defined as the loss of an intrauterine pregnancy after a gestational sac was identified on ultrasonography, but before 20 weeks of gestation. The rates of CPR, OPR/LBR, and early pregnancy loss were calculated for each euploid ET cycle, while the IR was calculated per euploid ET.
7. Statistical analysis
Continuous data were presented as the mean±standard deviation, and comparisons between groups were made using the independent-sample Student t-test. Categorical data were expressed as frequencies accompanied by percentages, and comparisons between groups were conducted using chi-square or Fisher exact tests. Data analysis was performed using SPSS version 12.0 (SSPS Inc.) and GraphPad Prism 9 (GraphPad Software Inc.), and p-values of less than 0.05 were considered to indicate statistical significance.
Results
1. Advanced maternal age
In the AMA group, PGT-A was performed on 944 biopsied embryos in 233 patients (mean age, 41.2 years; range, 38 to 45), of which 162 (17.2%) euploid embryos were analyzed. The euploid embryos selected for FET were transferred to 100 patients, while chromosomal abnormalities in all embryos precluded ET in the remaining 133 patients. The control group comprised 289 patients who underwent IVF and FET without PGT-A (mean age, 40.5 years; range, 38 to 44).
The CPR was significantly higher in the PGT-A group than in the control group (54.0% vs. 37.4%; odds ratio [OR], 1.967; 95% confidence interval [CI], 1.235 to 3.077; p=0.004). The PGT-A group also displayed a significantly higher IR (39.3% vs. 16.2%; OR, 3.342; 95% CI, 2.135 to 5.156; p<0.001), OPR/LBR per CPR (77.8% vs. 58.3%; OR, 2.500; 95% CI, 1.204 to 5.265; p=0.014), and OPR/LBR per ET (42.0% vs. 21.8%; OR, 2.598; 95% CI, 1.578 to 4.239; p<0.001). Early pregnancy loss was significantly less frequent among the PGT-A group than the control group (16.7% vs. 34.3%; OR, 0.384; 95% CI, 0.163 to 0.888; p=0.001); however, chemical pregnancy loss was similar between groups (5.6% vs. 7.4%; OR, 0.735; 95% CI, 0.204 to 2.564; p=0.661) (Table 5).
2. Recurrent implantation failure
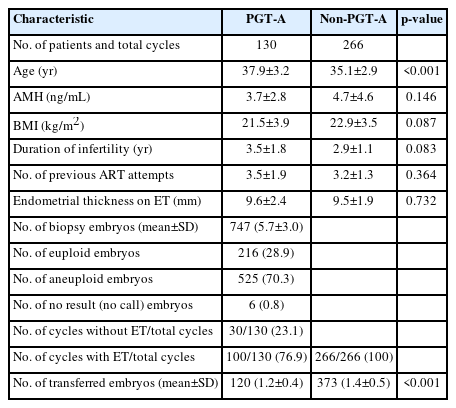
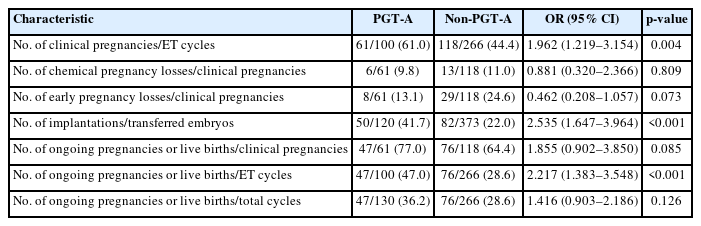
In the RIF group, PGT-A was performed on 747 biopsied embryos in 130 patients (mean age, 37.9 years; range, 30 to 44), of which 216 (28.9%) euploid embryos were analyzed. The euploid embryos selected for FET were transferred to 100 patients, while chromosomal abnormalities in all embryos precluded ET in the remaining 30 patients. The control group comprised 266 patients who underwent IVF and FET without PGT-A (mean age, 35.1 years; range, 27 to 39 years).
The PGT-A group exhibited a significantly higher CPR (61.0% vs. 44.4%; OR, 1.962; 95% CI, 1.219 to 3.154; p=0.004), IR (41.7% vs. 22.0%; OR, 2.535; 95% CI, 1.647 to 3.964; p<0.001), and OPR/LBR per ET (47.0% vs. 28.6%; OR, 2.217; 95% CI, 1.383 to 3.548; p<0.001) than the control group, whereas no significant difference was noted in OPR/LBR per CPR (77.0% vs. 64.4%; OR, 1.855; 95% CI, 0.902 to 3.850; p=0.085). Chemical pregnancy loss was similar between groups (9.8% vs. 11.0%; OR, 0.881; 95% CI, 0.320 to 2.366; p=0.809), and early pregnancy loss was lower in the PGT-A group than in the control group (13.1% vs. 24.6%; OR, 0.462; 95% CI, 0.208 to 1.057; p=0.073), but not to a statistically significant degree (Table 6).
3. Recurrent pregnancy loss
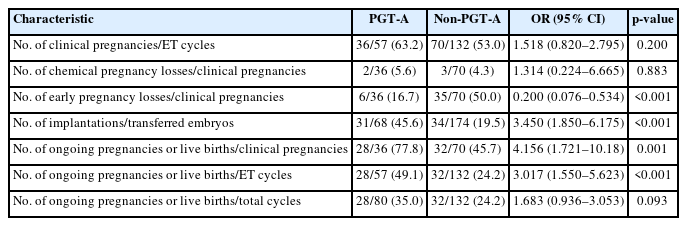
In the RPL group, PGT-A was performed on 407 biopsied embryos in 80 patients (mean age, 37.1 years; range, 28 to 45), of which 124 (30.5%) euploid embryos were analyzed. The euploid embryos selected for FET were transferred to 57 patients, while chromosomal abnormalities in all embryos precluded ET in the remaining 23 patients. The control group comprised 132 patients who underwent IVF and FET without PGT-A (mean age, 34.7 years; range, 24 to 39).
The CPR was higher in the PGT-A group than in the control group (63.2% vs. 53.0%; OR, 1.518; 95% CI, 0.820 to 2.795; p=0.200); however, this difference was not significant. The PGT-A group displayed a significantly higher IR (45.6% vs. 19.5%; OR, 3.450; 95% CI, 1.850 to 6.175; p<0.001), OPR/LBR per CPR (77.8% vs. 45.7%; OR, 4.156; 95% CI, 1.721 to 10.18; p=0.001), and OPR/LBR per ET (49.1% vs. 24.2%; OR, 3.017; 95% CI, 1.550 to 5.623; p<0.001) than the control group. Early pregnancy loss was significantly less frequent in the PGT-A group (16.7% vs. 50.0%; OR, 0.200; 95% CI, 0.076 to 0.534; p<0.001); however, the rate of chemical pregnancy loss was similar between groups (5.6% vs. 4.3%; OR, 1.314; 95% CI, 0.224 to 6.665; p=0.883) (Table 7).
4. Severe male factor infertility
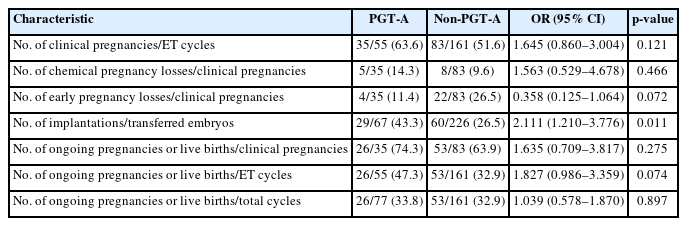
In the SMF group, PGT-A was performed on 408 biopsied embryos in 77 patients (mean age, 38.2 years; range, 30 to 44), of which 116 (28.4%) euploid embryos were analyzed. The euploid embryos selected for FET were transferred to 55 patients, while chromosomal abnormalities in all embryos precluded ET in the remaining 22 patients. The control group included 161 patients who underwent IVF and FET without PGT-A (mean age, 34.2 years; range, 23 to 39).
The IR was significantly higher in the PGT-A group than in the control group (43.3% vs. 26.5%; OR, 2.111; 95% CI, 1.210 to 3.776; p=0.011). In the PGT-A group, CPR (63.6% vs. 51.6%; OR, 1.645; 95% CI, 0.860 to 3.004; p=0.121), OPR/LBR per CPR (74.3% vs. 63.9%; OR, 1.635; 95% CI, 0.709 to 3.817; p=0.275), and OPR/LBR per ET (47.3% vs. 32.9%; OR, 1.827; 95% CI, 0.986 to 3.359; p=0.074) were higher, and early pregnancy loss was lower (11.4% vs. 26.5%; OR, 0.358; 95% CI, 0.125 to 1.064; p=0.072), than in the control participants; however, no significant differences were observed (Table 8).
Discussion
The ultimate goal of PGT-A in the context of IVF is to maximize the likelihood of a successful pregnancy and minimize the incidence of miscarriage through the selection of one or two chromosomally normal ETs. Patients at high-risk, including those with AMA, RIF, RPL, and SMF, have been documented to exhibit exceptionally high rates of aneuploidy [10], which accounts for the majority of implantation failures or miscarriages. In this study, we evaluated clinical outcomes for each indication by comparing euploid embryos from high-risk patients with AMA, RIF, RPL, or SMF who underwent aCGH or NGS against a control group with embryos selected based on morphological criteria.
1. Advanced maternal age
Embryonic aneuploidy, the incidence of which rises with maternal age, is the most common indication for PGT-A due to the elevated risk of miscarriage and implantation failure following IVF. Several studies have demonstrated the efficacy of PGT-A in patients of AMA. The selection and transfer of euploid embryos using aCGH-based PGT-A has been shown to improve IR and LBR in women aged 40 to 43 years [20]. In women aged 38 to 41 years, the selection of embryos using aCGH-based PGT-A resulted in a significantly higher LBR following PGT-A and a sharply lower pregnancy loss rate than in controls [21]. Furthermore, a meta-analysis reported that PGT-A improved LBR in women over 35 years old [22]. These findings support our results, which indicate that PGT-A led to a significantly higher IR and OPR/LBR per ET cycle, decreased the frequency of early pregnancy loss, and reduced the number of embryos transferred in the PGT-A group. This indicates the positive impact of euploid embryo selection and transfer using aCGH or NGS in patients of AMA. However, the OPR/LBR per total cycle was lower in the PGT-A group than in the control group, as embryonic aneuploidy precluded ET in 133 of 233 cycles. While most studies have reported beneficial effects of PGT-A in patients of AMA, some have reported opposing findings, with a meta-analysis of four RCTs [23] and a multicenter observational study [24] reporting no significant benefits.
2. Recurrent implantation failure
Kort et al. [25] demonstrated that patients with RIF exhibit significantly higher rates of aneuploidy than patients with normal fertility. Aneuploidy, which is closely linked to high rates of pregnancy failure, directly impacts embryo implantation and successful embryonic development, making it a primary cause of RIF. A multicenter prospective study revealed improvements in CPR (70.8% vs. 31.7%) and LBR (62.5% vs 31.7%) per ET in patients with RIF when PGT-A was used. However, the LBR per patient did not show a significant difference (35.7% vs. 26.0%) [26]. A study of advanced-age patients with RIF suggested that NGS-based PGT-A increases the likelihood of achieving a successful pregnancy [27]. Similarly, our findings revealed a significantly higher IR and OPR/LBR per ET cycle, along with a lower number of embryos transferred, in the PGT-A group than in control group. Moreover, the OPR/LBR per total cycle was higher in the PGT-A group than in the control group. Although this difference was not statistically significant, the findings offer a robust framework for comparison. This strong comparative framework underscores the positive impact of selecting and transferring euploid embryos using aCGH or NGS in patients with RIF.
3. Recurrent pregnancy loss
In accordance with the guidelines set forth by the American Society for Reproductive Medicine and the European Society for Human Reproduction and Embryology, RPL was defined in our study as two or more clinical pregnancy losses [16,17]. Given that aneuploidy is the cause of most early pregnancy losses, the use of PGT-A in conjunction with IVF is recommended for patients with RPL. A multicenter prospective study that evaluated the use of PGT-A in patients with RPL found no significant difference in the LBR per patient relative to controls (26.8% vs. 21.1%) [26]. In contrast, our findings revealed a significantly higher IR and OPR/LBR per ET in the PGT-A group compared with the control group, along with a significantly lower rate of early pregnancy loss. Furthermore, the OPR/LBR per total cycle was higher in the PGT-A group, although the difference was not statistically significant. This provides a more comprehensive framework for comparison. Our findings are corroborated by a recent study from the Society for Assisted Reproductive Technology Clinical Outcomes Reporting System, which assessed PGT-A in patients with RPL who had a good prognosis. This study demonstrated a significantly higher LBR (47.7% vs. 33.6%) and a significantly lower rate of spontaneous abortion compared to patients who did not undergo PGT-A (10.8% vs. 12.6%) [28]. Based on our results, the selection and transfer of euploid embryos using aCGH or NGS had a beneficial impact in patients with RPL.
4. Severe male factor infertility
Despite advancements in PGT-A with the introduction of new genetic testing platforms, the question of whether SMF should be considered an indication for PGT-A remains unresolved. The use of suboptimal sperm has been shown to increase aneuploidy in preimplantation blastocyst embryos [29]. In contrast, a large observational cohort study demonstrated that while SMF may adversely impact the early embryonic fertilization rate and developmental potential, it does not affect the euploidy rate or the implantation potential of the resulting blastocysts [30]. Furthermore, a study using NGS that included patients with SMF found no independent association between SMF and LBR per patient [31]. In our study, the use of PGT-A in the SMF group was associated with a significantly higher IR and a significantly lower mean number of transferred embryos than in the control group. However, in line with previous findings, we found no significant differences in the OPR/LBR per ET and OPR/LBR per total cycle between groups. While most studies have revealed no positive impact of using SMF as an indication for PGT-A, a recent retrospective study demonstrated that PGT-A for SMF (defined as azoospermia and a sperm concentration of less than 5×106/mL, and/or progressive motility of under 10%, and/or less than 4% morphologically normal spermatozoa) was associated with improved cumulative OPR and a significant reduction in early miscarriages [32]. However, when compared to a more robust control group, our results showed that using PGT-A in patients with SMF had no positive impact on OPR/LBR. As previously reported, SMF may not be independently related to clinical outcomes [31], and its effects necessitate further investigation with a larger dataset.
In the present study, among patients with AMA, RIF, or RPL after aCGH or NGS, PGT-A was associated with significant improvements in IR and OPR/LBR per ET relative to control group. Furthermore, the PGT-A groups experienced significantly lower rates of early pregnancy loss compared to the control groups among patients with RPL and AMA. These findings underscore the advantages of PGT-A for patients at an elevated risk of aneuploid embryos, given that chromosomal abnormalities are a primary contributor to early pregnancy loss and implantation failure. However, the application of PGT-A in IVF is restricted, as it may not be appropriate for all high-risk patients with early pregnancy loss and implantation failure due to various underlying causes. Consequently, patient selection is crucial. Specifically, in relation to the PGT-A outcomes of SMF, which offered a more reliable comparison group, it seems that SMF may not be independently linked to early pregnancy loss or implantation failure. However, it could be associated with other factors, such as characteristics of the partner.
This study did have certain limitations due to its retrospective design, despite efforts to match patients with similar profiles in the control groups. To ensure a robust assessment of PGT-A, we excluded patients who did not undergo ET from the control groups. Additionally, all patients in the RIF, RPL, and SMF control groups were under 40 years old, providing a stronger basis for comparison. Consequently, only minor differences were present in the demographic characteristics between the PGT-A and control groups. The lack of significant differences in OPR/LBR per total cycle among patients with RIF, RPL, and SMF may relate to these results. This, in turn, reinforces the robustness of the findings from these groups.
In conclusion, this study indicates that PGT-A is an effective tool associated with significant improvements in clinical outcomes among patients at heightened risk of aneuploid embryos, such as those with AMA, RIF, and RPL. Additionally, PGT-A mitigates the occurrence of multiple pregnancies by decreasing the number of ETs necessary for patients with AMA, RIF, and SMF. However, careful patient selection for this procedure is important for achieving positive outcomes. To validate our findings, further studies and meticulously designed RCTs are needed for each indication.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Author contributions
Conceptualization: JWK, CKP. Data curation: JWK, SYL. Formal analysis: JWK, SYL. Methodology: JWK, SYL. Project administration: CYH, JHL, CKP. Writing-original draft: JWK. Writing-review & editing: JWK, CYH, JHL, CKP.
Acknowledgements
The authors extend special thanks to their IVF laboratory colleagues and the clinicians at Maria S Fertility Hospital for their dedicated efforts throughout the study. Additionally, the authors are deeply grateful to their colleague Yejin Kim for providing valuable assistance with the statistical analysis using GraphPad Prism 9 software. This research did not receive any specific grant from funding agencies in the public, commercial, or non-profit sectors.