Detrimental impact of cell phone radiation on sperm DNA integrity
Article information
Abstract
Radiofrequency electromagnetic radiation (RF-EMR) from various sources may impact health due to the generation of frequency bands. Broad pulses emitted within frequency bands can be absorbed by cells, influencing their function. Numerous laboratory studies have demonstrated that mobile phones—generally the most widely used devices—can have harmful effects on sex cells, such as sperm and oocytes, by producing RF-EMR. Moreover, some research has indicated that RF-EMR generated by mobile phones can influence sperm parameters, including motility, morphology, viability, and (most critically) DNA structure. Consequently, RF-EMR can disrupt both sperm function and fertilization. However, other studies have reported that exposure of spermatozoa to RF-EMR does not affect the functional parameters or genetic structure of sperm. These conflicting results likely stem from differences among studies in the duration and exposure distance, as well as the species of animal used. This report was undertaken to review the existing research discussing the effects of RF-EMR on the DNA integrity of mammalian spermatozoa.
Introduction
The World Health Organization (WHO) defines infertility as the inability to achieve pregnancy after 12 months of unprotected sex [1]. Infertility is increasing in prevalence, affecting between 8% and 12% of couples of reproductive age [2]. This condition imposes psychological, social, and economic burdens on those affected. Research has indicated that paternal health and the quality of paternal gametes can influence the metabolic health and reproductive potential of offspring through epigenetic profiles [3]. Therefore, the study of paternal sex cells and their role in fertility is crucial. Various factors contribute to the development and progression of sperm cell disorders, which in turn increase the prevalence of male infertility. These factors include age [4], genetics [5], obesity [6], smoking [7,8], alcohol consumption [9], diseases such as varicocele [10], and exposure to electromagnetic radiation (EMR) [11-13]. Regarding the last of these factors, the proliferation and increased use of mobile telecommunication services have resulted in heightened exposure to radiofrequency electromagnetic radiation (RF-EMR) in daily life [14]. EMR can induce oxidative stress in sperm, causing concentrations of reactive oxygen species (ROS) to reach harmful levels; this can compromise the structure and function of spermatozoa, including various sperm parameters along with chromatin and DNA integrity. Research has demonstrated that the electromagnetic waves (EMWs) emitted by mobile phones can increase mitochondrial ROS in sperm, triggering oxidative stress and ultimately leading to DNA fragmentation [15,16]. De Iuliis et al. [15] found that RF-EMR, within the power density and frequency ranges of mobile phones, enhances mitochondrial ROS production in human spermatozoa. This can reduce sperm motility and vitality, stimulate DNA base adduct formation, and ultimately lead to sperm DNA fragmentation. Kang et al. [17] reported that radiation from mobile phones may cause structural and functional damage to the testes, alter semen parameters, and decrease epididymal sperm concentration. Sperm DNA fragmentation in the germ line has been linked to impaired fertilization, poor embryonic development, and high miscarriage rates [18]. In recent years, the rapid evolution of communication technology and the introduction of 5G technology have led to an increase in cell phone usage, resulting in higher exposure to associated RF-EMR. Therefore, it is crucial to gain a scientific understanding of the impact of mobile phone-generated RF-EMR on male fertility. Numerous studies have been conducted on this topic under various experimental conditions.
For this review, we drew upon existing studies to assess the impact of RF-EMR emitted from mobile phones on sperm DNA quality. Notable knowledge gaps include a detailed review of the mechanisms through which DNA damage occurs in human spermatozoa following exposure to mobile phone RF-EMR. Our objective was to explore the potential effects of this EMR on sperm chromatin and DNA structure, based on a thorough analysis of previous studies. Additionally, we present our perspectives on this subject to establish a scientific foundation for future research.
Materials and methods
We conducted a literature search using the ISI Web of Science (http://apps.lib.wosg.ir/WOS), MEDLINE–PubMed (http://www.ncbi.nlm.nih.gov/PubMed), Scopus (https://www.scopus.com), and Google Scholar (https://scholar.google.com/) databases, covering the period from the inception of online indexing to January 2023. Searches were conducted using medical subject headings (MeSH) and non-MeSH keywords, including “cell phone,” “sperm,” “oxidative stress,” “reactive oxygen species (ROS),” “DNA damage,” and “apoptosis.” The search was limited to original articles written in English that examined the impact of mobile phone radiation on chromatin structure, DNA, and sperm apoptosis. We excluded any studies that explored the effects of mobile phone radiation on parameters other than those specified.
Biophysical mechanism of EMR emitted by cell phones
EMR can be categorized into ionizing and non-ionizing waves. Non-ionizing waves, which have a low frequency range (800 to 2,200 MHz), cannot break the chemical bonds present in biological systems. In contrast, ionizing waves, at frequencies of around 1,000,000 MHz, are sufficiently intense to break chemical bonds [12]. Mobile phones are among the devices that generate non-ionizing EMR. The EMR emitted from cell phones is intercepted by various receivers, including the body. Human bodies function like antennas, absorbing these waves and transforming them into alternating currents. When a cell phone is used for conversation, it converts the sound wave dispatched by the transmitter into a sine wave. This electrical sine wave generates an electromagnetic field around it. EMR is produced by the oscillation of electric current, and this radiation is emitted in the form of radio waves [12]. RF-EMR has the potential to be absorbed by the body. This absorption can be quantified using a standard unit known as the specific absorption rate (SAR), measured in W/kg. The maximum legally permissible SAR for a cell phone device is capped at 1.6 W/kg [19].
Mechanism and biological effects of cell phone usage on male fertility
RF-EMR emitted from mobile phones can induce biological changes in spermatozoa through two mechanisms: thermal and non-thermal effects. The EMR absorbed can transform into heat, resulting in thermal effects. The degree of heat generated depends on the SAR and the power density of the EMR. If the heat produced exceeds 100, it is plausible to anticipate the onset of biological changes [20]. Spermatogenesis in the testes occurs at a temperature approximately 2 °C lower than the body temperature. Consequently, thermal effects can cause DNA damage, germ cell apoptosis, and disruption of the seminiferous tubule epithelia [21].
The non-thermal effects of EMWs are manifested through various mechanisms, including oxidative stress, alterations in sperm membrane potential, signaling pathways, and changes in sperm proliferation and apoptosis [12]. Sperm is particularly susceptible to oxidative stress due to factors such as the absence of an antioxidant system and the presence of targets for oxidative damage, including polyunsaturated acids and DNA structure. Furthermore, the existence of ROS generators, such as mitochondria and the nicotinamide adenine dinucleotide hydrogen (NADH) oxidase of the plasma membrane, contribute to this sensitivity [22]. Exposure to EMR activates the plasma membrane NADH oxidase, leading to the production of ROS. In cases of chronic exposure to such radiation, the quantity of ROS generated surpasses the antioxidant capacity, resulting in oxidative stress [14]. Oxidative stress is a primary contributor to sperm DNA damage.
Impact of electromagnetic waves on male sex cells
Several studies have affirmed the association between DNA damage from both internal and external sources and the disruption of mammalian reproductive structure and function following exposure to waves. Leydig cells, among male sex cells, are particularly sensitive to EMWs. Any disturbance in these cells can lead to alterations in spermatogenesis [23]. EMW can influence the function of Leydig cells by reducing hormone secretion, which in turn leads to changes in cell proliferation during spermatogenesis. Several studies have explored the impact of EMW (860 to 915 MHZ) on testicular structure. The findings from these studies suggest a reduction in the diameter of the seminiferous tubules and the thickness of the epithelium, as well as a decrease in the weight of the testicular organs [24]. Epidemiological studies have demonstrated that mobile phones can negatively affect semen quality and result in a decrease in sperm functional parameters. These studies have reported that exposure to waves is linked with a decrease in sperm count, motility, viability, and morphology. Most notably, they have found an increase in sperm chromatin damage and apoptosis [25-30].
Effect of EMW on sperm DNA structure
The unique architecture of sperm is defined by its simple cell structure, the lack of substantial cytoplasm, and a minimal number of cytosolic organelles, with the exception of the nucleus, flagellum, and mitochondria. The sperm membrane is rich in unsaturated fatty acids, which are highly sensitive to ROS [31]. This structure is susceptible to electroporation, a process triggered by stress from external waves, which results in the formation of a large hole in the membrane [32]. This phenomenon leads to alterations in the homeostasis of ions, including calcium, and ultimately results in sperm inefficiency. Conversely, the sperm membrane possesses a redox system akin to NADH oxidase traversing the membrane [33]. The activation of membrane NADH oxidase due to EMW stimulates the cell and leads to ROS production [34]. While the sperm cytoplasm can be a site for ROS production, the primary site of ROS production is the mitochondria [35]. Exposure to EMR enhances mitochondrial NADH oxidase activity, which is marked by the formation of oxidative base adducts and DNA fragmentation [15]. Damage to the chromatin DNA structure caused by ROS action and oxidative stress conditions is typically attributed to indirect DNA damage (Figure 1). In contrast, the direct theory posits that the two strands of DNA function like a cable that carries induced electric currents from various directions. These currents generate electromagnetic forces around each strand in opposing directions, leading to a progressive repulsion effect between the two strands. Consequently, the two DNA chains gradually separate, and in extreme cases, the two DNA strands break and become fragmented [36]. EMW, in conjunction with DNA oxidative damage and DNA fragmentation, inflict damage on this structure. ROS induced by waves diminish the activity of antioxidant enzymes, such as superoxide dismutase, glutathione peroxidase, and catalase, thereby inducing a state of oxidative stress [37]. The deoxyguanosine (8-oHdG) index and the tunnel test are employed to assess sperm DNA oxidative damage and DNA fragmentation, respectively.
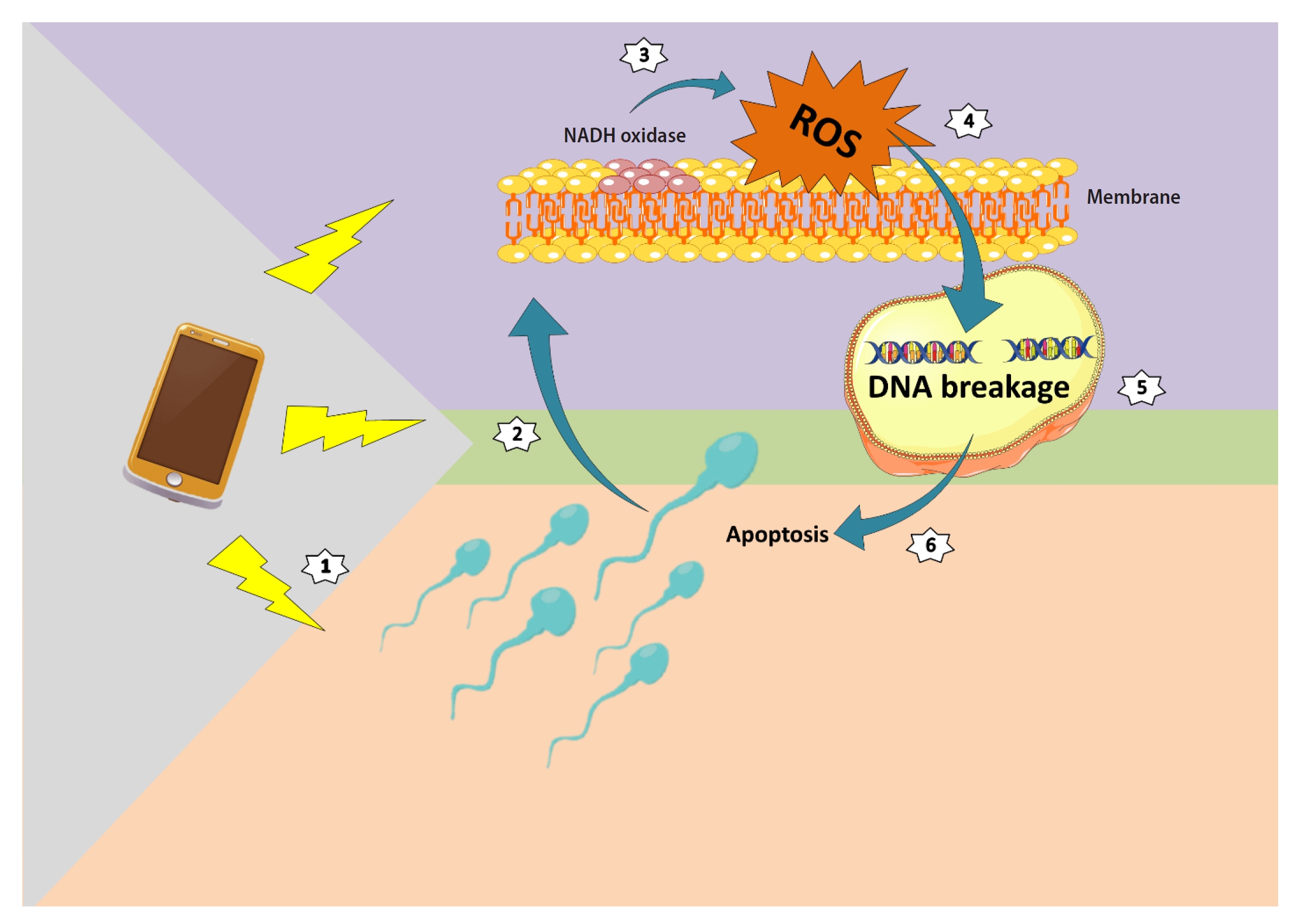
Electromagnetic waves emitted by mobile phones stimulate nicotinamide adenine dinucleotide hydrogen (NADH) oxidase in the plasma membrane, which in turn affects the integrity of the sperm nucleus. Within the nucleus, the structure of the DNA deteriorates, ultimately guiding the cellular structure towards apoptosis. ROS, reactive oxygen species.
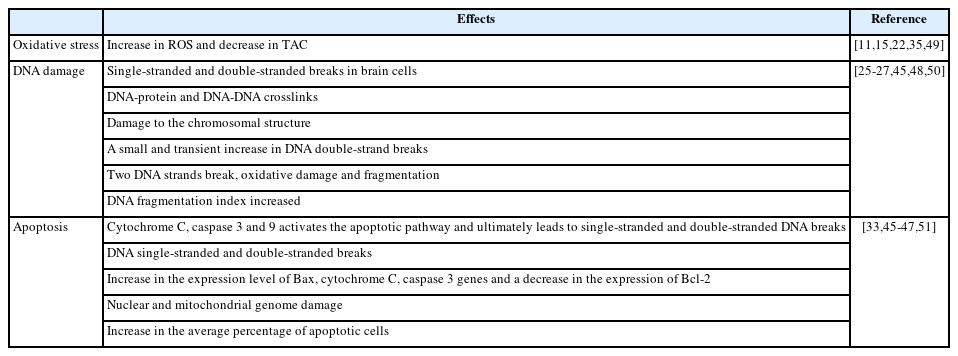
Several studies have evaluated the expression of 8-oHdG and utilized the tunnel test to demonstrate that exposure to EMW can lead to an increase in mitochondrial ROS production and DNA breakage, with these effects intensifying as SAR levels rise [11,38,39]. In a study conducted by Zalata et al. [40], sperm samples from individuals with asthenozoospermia, asthenoteratozoospermia, or oligoasthenoteratozoospermia (OAT) were exposed to mobile phone waves. The researchers observed a significant increase in sperm DNA fragmentation in the OAT group that had been exposed to mobile phones [40]. Veerachari and Vasan [41] also investigated the potential adverse effects of cell phone radiation on human ejaculates. The samples were exposed to a frequency of 900 MHz for 60 minutes. The evaluation of DNA integrity using acridine orange and halo sperm tests revealed a significant increase in the DNA fragmentation index in the group exposed to mobile phones, compared to the control group [41]. In a separate study, pure male semen samples were exposed to mobile phone waves for a duration of 3 hours. The sperm chromatin dispersion test revealed evidence of DNA fragmentation in the exposed group, compared to the control group [42]. Rashad et al. [43] exposed samples from infertile men to EMWs for varying time intervals of more than 2, 4, and less than 4 hours per day. The Comet test results indicated DNA damage and changes in the sperm DNA strands, with the exposure group that was exposed for more than 4 hours per day showing more damage compared to the other groups [43]. However, not all findings support the destructive effect of cell phone waves on DNA. Some studies have shown that exposure of sperm cells to 900 MHz cell phone waves does not alter DNA integrity [44]. Similarly, Agarwal et al. [11] reported no significant change in DNA damage between the group exposed to mobile phone waves and the control group. These conflicting results regarding DNA damage may be attributed to the source of sperm preparation and the test protocols used, such as exposure time, frequency of EMWs, SAR, and other factors (Table 1) [11,15,22,25-27,33,35,45-51].
DNA damage and apoptosis
Apoptosis and DNA damage due to oxidative stress can potentially follow two pathways. According to one theory, oxidative stress, resulting from the accumulation of ROS, leads to an increase in the rate of single-stranded and double-stranded DNA breaks. If these breaks are not properly repaired, it can trigger apoptosis [45]. Another theory suggests that the accumulation of ROS, caused by mitochondrial function through cytochrome C, caspase 3 and 9, activates the apoptotic pathway, ultimately leading to single-stranded and double-stranded DNA breaks [46]. In a study by Liu et al. [47], which focused on sperm apoptosis after exposure to 900 MHz EMWs, a significant relationship was found between apoptosis and changes in the expression of B-cell lymphoma 2 (Bcl-2) associated X (Bax), Bcl-2, caspase-3, and cytochrome C genes. The results of RT-PCR and Western blot analyses showed that an increase in ROS and a decrease in total antioxidant capacity were associated with a significant increase in the expression level of Bax, cytochrome C, caspase 3 genes, and a decrease in the expression of Bcl-2 in the sperm of exposed mice [47]. Aitken et al. [48] also discovered that exposing epididymal sperm of mice to 900 MHz EMWs for 7 days resulted in nuclear and mitochondrial genome damage. Following this damage and the sperm's limited ability to repair DNA damage after spermiation, the sperm cells underwent apoptosis [48]. In another study, based on the theory that EMWs can increase scrotal temperature, with detrimental effects on sperm production and maturation, it was suggested that these adverse conditions could cause DNA damage and apoptosis of germ cells [52]. Additionally, Kesari and Bahari [49] exposed human sperm cells to 900 MHz EMWs for 2 hours a day over 45 days. Their data indicated a correlation between an increase in the expression of caspase 3 and subsequent apoptosis [49]. In a separate study, rats were exposed to mobile phone EMWs with 0.9 SAR for 35 days. Mice in the exposure group exhibited a significant increase in the average percentage of apoptotic cells [53]. Moreover, studies by Doostabadi et al. [2] and Agarwal et al. [29] supported the substantial impact of EMWs on reducing semen quality. They demonstrated that, in addition to reducing sperm functional parameters, EMR was associated with increased apoptosis, which may contribute to male infertility [29,54]. However, despite the studies confirming the destructive effect of EMWs on sperm apoptosis, it is important to consider that the extent of damage to sperm structure and induction of apoptosis will depend on the cell type and the nature and duration of exposure to EMWs.
Conclusion
EMW can induce oxidative stress, which subsequently leads to disorders such as reduced mobility, morphological changes, acrosome disturbances, and ultimately, damage to the nucleus and genetic material. This oxidative damage to DNA can result in the breakdown of both single-stranded and double-stranded DNA structures, culminating in fragmentation. If the DNA is not repaired and the damage accumulates, the sperm may undergo apoptosis. Damage to the sperm genome can ultimately impact fertility, potentially leading to infertility. Therefore, it is advisable to limit daily exposure to these sources to prevent irreversible damage caused by EMWs. Many men carry their cell phones in their trouser pockets or clipped to their belts, and the use of Bluetooth can increase their susceptibility to RF-EMR exposure. This exposure can induce changes in sperm quality through oxidative stress, potentially leading to infertility. Agarwal et al. [11] suggested that carrying a cell phone in a pocket could lead to a decline in sperm quality. However, it is important to note that the phone and male reproductive organs are separated by multiple tissue layers. Therefore, extrapolating these in vitro effects to real-life conditions requires further studies [11].
In July 2021, the European Parliament commissioned a research report titled “Health impact of 5G.” The report concluded that the commonly used RF-EMFs are likely carcinogenic to humans and have a definitive impact on male fertility. It also suggested potential adverse effects on the development of embryos, fetuses, and newborns. To mitigate these adverse effects, the organization proposed several strategies. These include favoring non-wireless connections, increasing distance from the source of RF-EMFs, switching off devices when not in use, and practicing safe phone usage [55].
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Acknowledgements
Conceptualization: YK, MAK. Visualization: YK. Writing-original draft: MAK, FD. Writing-review & editing: YK, MAK, FD, MS.