Effects of a short abstinence period on sperm quality in oligozoospermic men
Article information
Abstract
Objective
The aim of this study was to compare semen parameters and sperm DNA fragmentation (SDF) and explore the relationship between semen parameters and SDF between 2 and 7 days of abstinence and a short abstinence period (within 4 hours) in oligozoospermic infertile patients.
Methods
Two semen samples were collected from infertile oligozoospermic men (n=34) after an abstinence period of 2 to 7 days and within 4 hours, respectively. Sperm parameters were compared between the two abstinence duration groups, including semen volume, sperm concentration, total sperm count, sperm motility, total motile sperm count (TMSC), morphology, and SDF.
Results
The semen volume, concentration, and total sperm count were significantly decreased after 4 hours of abstinence than after 2 to 7 days of abstinence, with median differences of 1.2 mL (p<0.001), 2×106/mL (p=0.011), and 9.6×106/ejaculation (p<0.001), respectively. TMSC was significantly lower after a short abstinence, with a median difference of 4.24×106/ejaculate (p<0.001). However, there were no significance differences in the percentage of motility, the SDF, and the percentage of sperm with normal morphology. Interestingly, volume, concentration, total sperm count, sperm motility, and SDF, but not TMSC, exhibited significant linear correlations between the two abstinence groups in univariate regression analysis, except for TMSC.
Conclusion
In oligozoospermic men, the volume, concentration, and total sperm count were significantly lower after a short abstinence period, but without adverse effects on sperm motility and SDF.
Introduction
Male factor infertility is the sole cause of infertility in approximately 20% of infertile couples and an essential contributing factor in another 20% to 40% of couples with reproductive failure [1]. In the initial evaluation, at least one proper semen analysis should be performed. If an abnormality is found, another semen analysis should be performed after at least 4 weeks [2]. Oligozoospermia is the most common abnormality and is typically a crucial contributing factor in men classified as infertile [3]. To solve infertility problems, assisted reproductive technologies (ARTs), such as intrauterine insemination (IUI), in vitro fertilization (IVF), and intracytoplasmic sperm injection (ICSI), have been developed. These procedures require sperm preparation to concentrate motile spermatozoa. Progressive motility and the total motile sperm count (TMSC) are the initial sperm characteristics most closely related to pregnancy. IUI is an effective therapy for male factor infertility when initial sperm motility is ≥30%, and the TMSC is ≥5×106 per ejaculate [4]. IVF is preferable to IUI when the initial values are lower because of its higher effectiveness and cost-effectiveness. ICSI generally serves as a robust bypass procedure instead of a first-line treatment, and it has dramatically improved the fertility prospects in patients with oligozoospermia [5]. However, standard semen analysis may not completely provide all information to evaluate male fertility status, as 15% of patients with male factor infertility were found to have normal semen analysis results [6]. Therefore, additional tests should be performed in addition to using the results of the semen analysis alone. Extensive research has been conducted during the past decade is the integrity of sperm DNA. Several studies have shown that sperm DNA fragmentation (SDF) had a statistically significant negative impact on the chance of pregnancy [7].
Human sperm is produced in the seminiferous tubules and stored in the epididymis for future release. The epididymal transit time has been estimated to range from 2 to 11 days. Variation is influenced by the frequency of ejaculation [8]. During epididymal transit and storage, spermatozoa are significantly exposed to reactive oxygen species (ROS) [9]. Prolonged exposure to ROS results in an alteration of sperm function and fertilizing capacity. They also affect the sperm genome, causing high frequencies of single‐ and double‐strand DNA breaks [10]. The World Health Organization (WHO) recommends 2 to 7 days of abstinence before collection for standard semen analysis [11]. However, recent studies have suggested that shorter abstinence is associated with improved ART outcomes [12-16]. A large cohort study in normozoospermic subfertile men showed that longer abstinence was associated with increased ejaculate volume, concentration, total sperm count, and TMSC. However, in oligozoospermic men, longer abstinence time was not associated with improvements in semen parameters, except for ejaculate volume [17]. Prolonged sexual abstinence can have a negative impact on motility, viability, and SDF [18]. However, a significant increase in total and progressive motility was observed after a short abstinence period of 4 hours [19]. One day of abstinence improved sperm quality by protecting against ROS damage and a higher total seminal antioxidant capacity [20]. There is a lack of consensus on the exact impact of the abstinence period on the conventional and functional parameters of sperm. Thus, the effect of sexual abstinence on sperm parameters is still debatable [18,21-23].
The association between short abstinence and semen quality in oligozoospermic men has been evaluated in some studies, but the results have remained inconclusive. Therefore, we conducted a prospective experimental study in oligozoospermic men to compare sperm parameters and SDF in semen samples after 2 to 7 days of abstinence and within 4 hours of abstinence and explore the relationship between semen parameters and SDF between the two abstinence periods.
Methods
1. Study population and sample size
This single center prospective experimental study enrolled 42 oligozoospermic men aged 20 to 45 years with infertility problems visiting the Assisted Reproductive Technology Clinic, Ramathibodi Hospital, Bangkok, Thailand, from February 2021 to February 2022. The study was approved by the Committee on Human Rights Related to Research Involving Human Subjects at Ramathibodi Hospital, Mahidol University (MURA 2020/1895). Written informed consent was obtained from all patients.
2. Inclusion criteria
Men who met all inclusion criteria were invited to participate in the study.
(1) Men aged 20 to 45 years with infertility that had lasted for more than 1 year at the time of screening.
(2) At least one previous semen analysis result with a sperm concentration less than 15×106/mL with an abstinence period of 2 to 7 days.
(3) A semen volume of at least 1.5 mL per ejaculation at the screening.
(4) No history of previous testicular/penile surgery or vasectomy.
(5) No history of cancer, no history of radiation therapy, or chemotherapy.
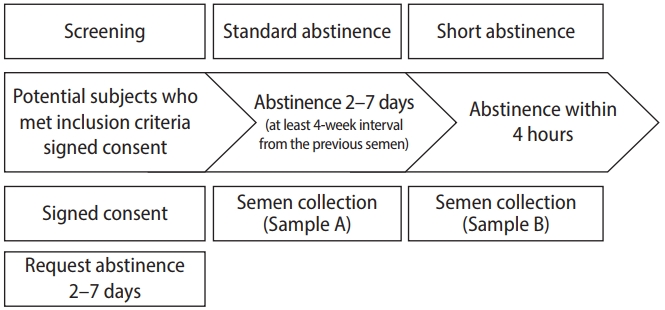
Volunteers who met all inclusion criteria were asked to abstain for 2 to 7 days to collect study samples. Furthermore, the study sample collection had to be collected for at least 4 weeks from the previous semen analysis. Semen and SDF analyses were performed from semen after the abstinence for 2 to 7 days (referred to as the standard abstinence sample or sample A) and from semen collected within a 4-hour interval from sample A (referred to as the short abstinence sample or sample B) (Figure 1).
3. Exclusion criteria
The volunteers were excluded from the study analysis if they had one of the following.
(1) Sperm concentration ≥15×106/mL when repeated at least 4 weeks after a previous semen analysis.
(2) Inability to ejaculate by masturbation.
(3) Azoospermia from sample A.
4. Semen collection and analysis procedure
Semen samples were collected from the volunteers by masturbation and provided routine information on the duration of abstinence to technicians. After liquefaction, the semen volume and pH were measured. Sperm motility analysis was performed on a 10-μL drop on a glass slide with a 22×22 mm coverslip for two replicates. Sperm motility was analyzed with phase-contrast optics at ×200 magnification with an eyepiece reticle. At least 200 sperm were evaluated in each replicate to determine the percentage of different motility categories according to the WHO 2010 guideline [11].
Sperm concentration measurement was performed by diluting a 50-μL semen sample with fixative. Sperm counting was then performed using an improved Neubauer hemocytometer. At least 200 spermatozoa per replicate were counted and compared whether the difference is acceptable or not according to WHO 2010 standard. If so, proceed to calculate the concentration in spermatozoa per mL; if not, prepare a new dilution.
Sperm morphology was evaluated by eosin/methylene blue staining (Dip Quick Stain; SE Synergist) using bright field optics at ×1,000 magnification with an oil immersion microscope based on Kruger's strict criteria.
5. SDF assessment using a TUNEL assay and flow cytometry
SDF was evaluated using a terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay with an APO-DIRECT Kit (BD Biosciences), as described by Sharma et al. [24]. Spermatozoa within the propidium iodide (PI)/RNase solution were analyzed by flow cytometry (BD FACSVerse Flow Cytometer). The output data were imported and analyzed using BD FACSuit software with a 488 nm argon laser as the light source. Two dyes were used: PI, which stains total DNA, and fluorescein isothiocyanate (FITC-dUTP), which stains broken DNA. PI fluoresces at about 623 nm and FITC at 520 nm. The results were expressed as the percentage of SDF. The Supplementary Figure 1 shows the measurement of SDF.
6. Statistical analysis
Statistical analyses were performed using STATA Statistics for Windows version 14.0 (StataCorp LLC). For continuous variables, the mean with standard deviation (SD) or median with interquartile range (IQR) were used for data presentation as appropriate. Frequencies with percentages were used to describe categorical data. The paired t-test or Wilcoxon signed-rank test was used to compare continuous data from the short and 2- to 7-day periods of abstinence. Linear regression analysis explored the linear relationship between parameters with both abstinence periods. The level of statistical significance was set at p<0.05.
Results
1. Baseline characteristics of semen parameters at screening
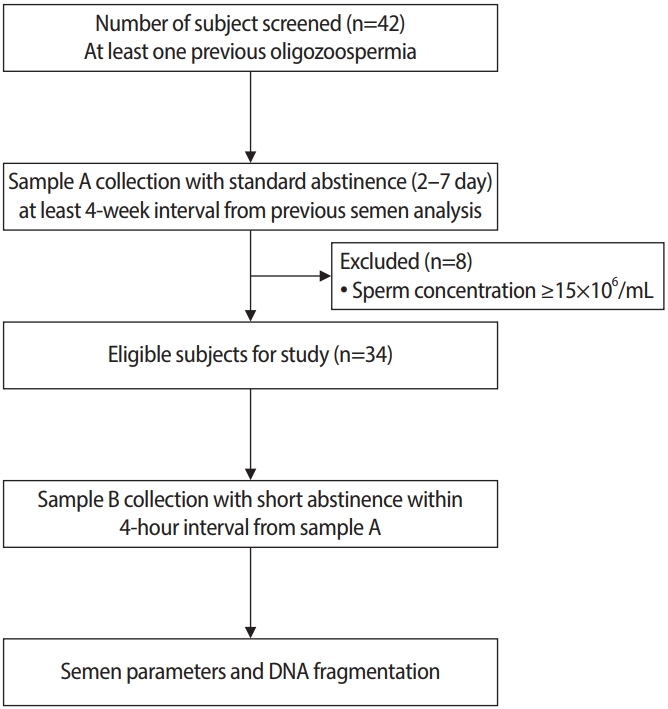
Forty-two men with at least one previous examination indicating oligozoospermia who met the study inclusion were invited and consented to participate in the study. Eight volunteers were excluded from the study analysis because sample A showed a sperm concentration ≥15×106/mL. Therefore, 34 volunteers were included in the study (Figure 2).
The mean±SD age of the oligozoospermic men in this study was 37.08±5.05 years, with the median of semen volume, concentration, and total sperm count of 2.75 mL (IQR, 1.6 to 3.4), 6×106/mL (IQR, 3 to 10), and 13.6 ×106/ejaculate (IQR, 7.25 to 26.6), respectively. The mean±SD of total motility was 46.64%±15.62%. The baseline characteristics of the semen parameters are shown in Table 1.
2. Comparison of semen parameters and SDF between 2 and 7 days and short abstinence
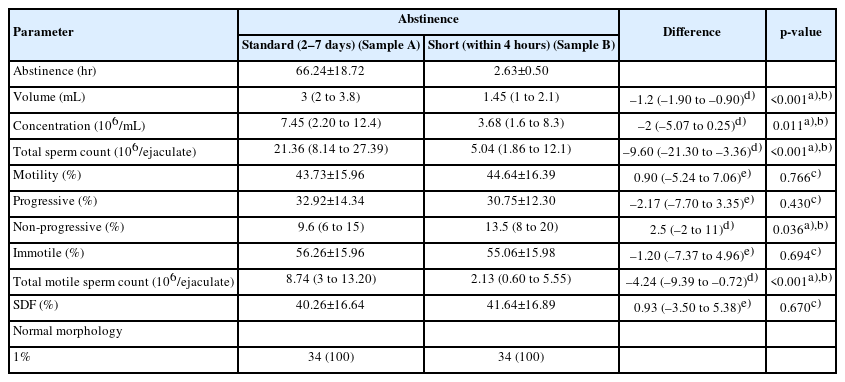
Semen volume, concentration, and total sperm count were significantly lower after the short abstinence period than after 2 to 7 days of abstinence. The median differences in the volume, concentration, and total sperm count after the short abstinence period were 1.2 mL (p<0.001), 2×106/mL (p=0.011), and 9.6×106/ejaculate (p<0.001), respectively. The percentage of non-progressive motility was significantly higher after the short abstinence period (p<0.036), while there was no difference in the percentage of immotile sperm (p=0.694). The TMSC was significantly lower after the short abstinence period than after 2 to 7 days of abstinence, with a median difference of 4.24×106/ejaculate (p<0.001). There were no significant differences in the percentage of total motility, the percentage of SDF, and the percentage of normal morphology (Table 2).
3. Relationship of semen parameters and SDF between 2 and 7 days and short abstinence
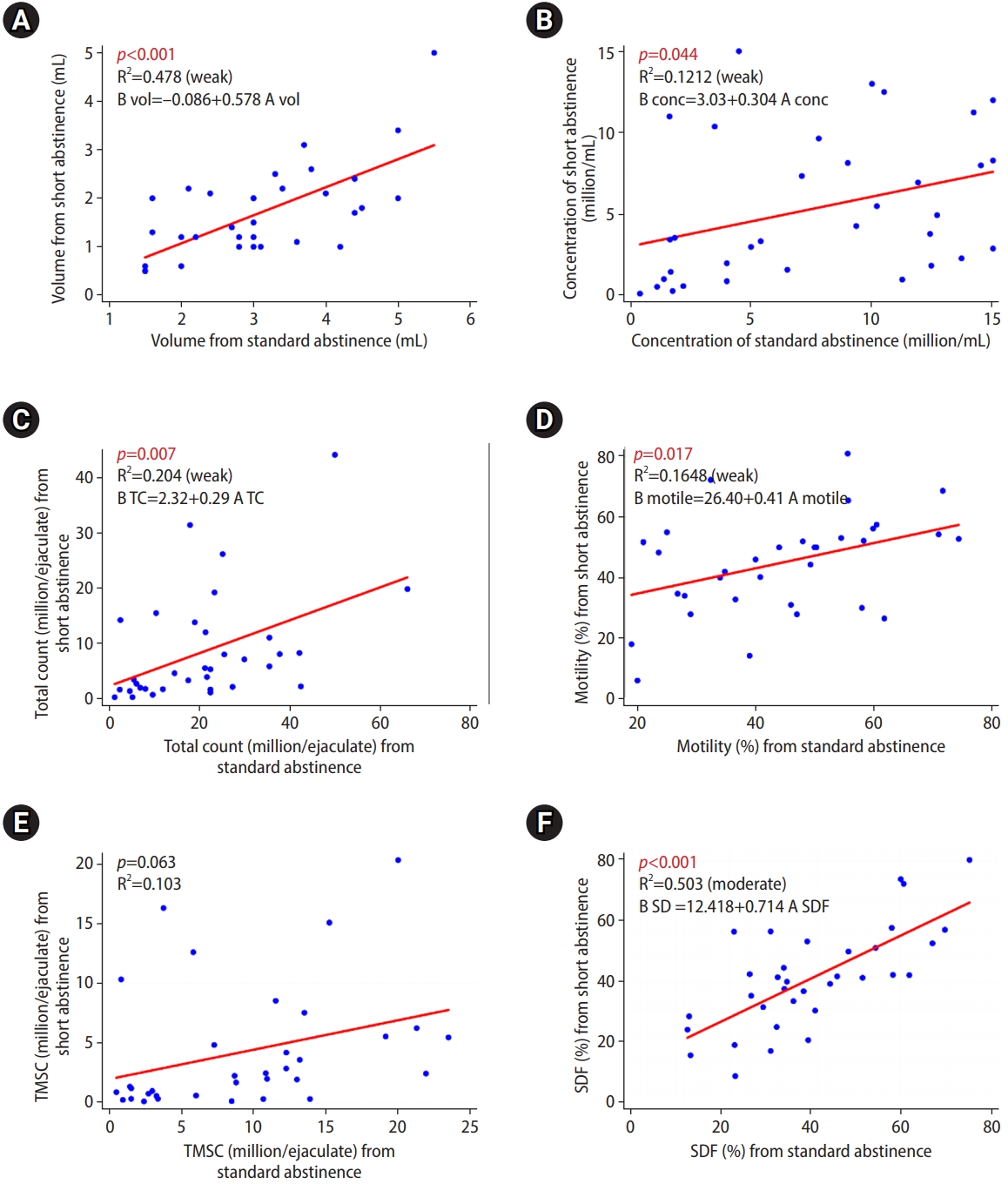
Univariate regression showed significant linear relationships of volume, concentration, total sperm count, sperm motility, and SDF between the samples from 2 to 7 days of abstinence and those from the short abstinence period, except for TMSC (Figure 3). The Supplementary Figure 2 shows the SDF results of each semens.
Discussion
In this study, in oligozoospermic men, a short abstinence period (within 4 hours) was associated with lower semen volume, concentration, and total sperm count than the values observed after 2 to 7 days of abstinence. The median differences in semen volume, concentration, and total sperm count in short abstinence were 1.2 mL, 2×106/mL, and 9.60×106/ejaculate, respectively. Furthermore, there were significant linear relationships in volume, concentration, and total count between the two abstinence periods. Hence, repeated semen collection within 4 hours is likely to provide comparable or lower results in terms of semen volume, sperm count, and sperm motility. Most previous studies in normozoospermic men found that shorter abstinence resulted in lower volume, concentration, and total sperm count [17,19,21,25,26]. However, studies in men with oligozoospermia have inconsistent regarding whether shorter abstinence decreases or increases semen volume, concentration, and total count [15,26,27]. Studies in infertile men have shown that the duration of abstinence had a statistically significant favorable influence on semen volume, sperm concentration, and total sperm count [13,14,18]. The results of the present study appeared to be consistent with most studies in normozoospermic and infertile samples, showing that shorter abstinence resulted in lower semen volume, concentration, and total sperm count. The reduction in sperm concentration and total count in the short abstinence ejaculate may be due to the lack of time to transfer spermatozoa from the more proximal sections of the epididymis to the cauda and vas deferens, as well as semen volume, the majority of which is produced in the seminal vesicles and prostate gland.
This study showed that the percentage of non-progressive motility was significantly higher in the short abstinence group, while there was no significant difference in the percentage of immotile sperm. The TMSC was significantly lower in the short abstinence group than after 2 to 7 days of abstinence, with a median difference of 4.24×106/ejaculate. Univariate regression also showed significant linear relationships of sperm motility between 2 and 7 days and short abstinence, but no linear relationship of TMSC was detected. In terms of motility, the results of the present study differ from those of most previous studies. Previous studies in normozoospermic men showed a significant decrease in the percentage of sperm motility on days 11 to 14 of sexual abstinence compared with abstinence of fewer than 11 days [23]. In other words, shorter abstinence was associated with better sperm motility. Short abstinence (4 hours) was associated with significantly higher values of the total and progressive motility and velocity parameters [19]. Short abstinence (2 hours) showed higher velocity, progressiveness, and hyperactivation [25]. In addition, 1 day of abstinence showed better sperm motility than 4 days of abstinence [28]. However, another study found no difference in total motility and TMSC between 3–5 days and 18–30 hours of sexual abstinence [29]. Previous studies of patients with oligozoospermia or oligoasthenoteratozoospermia showed better motility with shorter abstinence periods [15,26,27]. A maximum mean sperm motility of 30.3% was observed after 1 day of abstinence [23]. Short abstinence (40 minutes) improved progressive grade A motility [27]. The sperm motility and TMSC of the second ejaculation (after 30 to 60 minutes of abstinence) were significantly higher than those of the first ejaculation (after 3 to 5 days) [15]. Sexual abstinence of less than 24 hours showed the highest mean percentage of progressively motile sperm [26]. The significant increase in non-progressive motility after a short abstinence period from this study is inconsistent with the findings of most previous studies. In oligozoospermic men, the sperm transport time through the epididymis was three times longer than in normozoospermic men. In addition, it has been suggested that some patients with supposed idiopathic testicular failure might have a partial obstruction [23]. Therefore, spermatozoa may be exposed to a high level of ROS for a more extended period in the genital tract, which might have a deleterious effect on sperm motility. However, there were remarkable variations between the duration of the short abstinence periods and the control group among the previous studies compared to our study.
This study included only oligozoospermic men; all samples from the 2 to 7 days and short abstinence periods had a 1% proportion of normal morphology according to Kruger's strict criteria. A large study in 1,621 normozoospermic samples revealed no difference in morphology according to abstinence time [26]. Most studies in men with oligozoospermia have shown an increase in normal morphology with short abstinence [15,23,27]. However, a study in 416 oligozoospermic samples did not find a significant difference in the percentage of normal sperm morphology during different abstinence times. Nonetheless, an abstinence period of less than 24 hours was associated with the highest mean percentage of normal sperm morphology [26]. This study could not compare morphology because all oligozoospermic samples had 1% proportions of normal morphology. A high level of SDF should cause this result in all samples; furthermore, we analyzed a small number of samples. The percentage of normal sperm morphology showed a significant negative correlation with the percentage of SDF [30].
There was no significant difference in the percentage of SDF in this present study between the short abstinence period and 2 to 7 days of abstinence. Most studies in normozoospermic men showed an improvement in SDF with short abstinence periods [18,21,22,29,31]. However, a study of 100 normozoospermic men found no differences in SDF between an abstinence period of 4 days and 4 hours [19]. A study that included both normozoospermic and oligozoospermic men showed lower SDF after a short abstinence period. However, the study did not perform a subgroup analysis for each group [31]. The results from the present study in terms of SDF are inconsistent with previous study results. Different methodologies for detecting SDF (e.g., TUNEL assay, the comet assay, the sperm chromatin structure assay, and the sperm chromatin dispersion test) may also be responsible for these contradictory findings. Since the etiology of SDF is multi-factorial, involving intrinsic and/or extrinsic factors [32], a persistently high level of SDF despite short abstinence in this study might reflect severe damage to spermatozoa due to problems with the sperm chromatin packaging process during spermatogenesis.
1. Strengths
Most previous studies investigating the influence of ejaculatory abstinence on semen parameters were retrospective and based on the results of a single semen analysis. In this study, we conducted prospective research on semen parameters in the same participants who had confirmed oligozoospermia by repeating the semen analysis after at least 4 weeks to avoid enrolling individuals with significant discrepancies in sperm concentration.
There may have been substantial variation in sperm counting chambers and counting techniques in many studies, which could have led to significant overestimations or underestimations of sperm motility and concentration, especially in oligozoospermic samples [11,33]. Therefore, we performed semen analysis based on the standard WHO 2010 guideline [11], which provides the most accurate results. We excluded men with a semen volume of less than 1.5 mL/ejaculate associated with ductal obstruction or incomplete samples. The TUNEL assay, a direct assay to assess DNA fragmentation, was used with flow cytometry; this method provides objective and accurate results with minimal interobserver variability, can be performed on a few sperm, and has a high level of experimental repeatability.
2. Limitations
The results of this study were obtained from a single center and with a relatively low number of men. In addition, the participants in this study were 45 years old or younger. Therefore, the results could not be generalized to oligozoospermic men >45 years of age. Furthermore, we did not have data on ROS testing and other functional semen parameters. Additionally, sperm parameters, as intermediate outcomes for infertility treatment, may not reflex the pregnancy outcomes.
3. Conclusions
This study in oligozoospermic men showed that concentration, volume, total count, and TMSC after a short abstinence period were significantly lower than after 2 to 7 days of abstinence. There were no significant differences in the percentage of total sperm motility and SDF between 4 hours and 2–7 days of abstinence. Significant linear relationships were found for volume, concentration, total sperm count, sperm motility, and SDF, but not TMSC, between both groups in the univariate regression analysis. A short abstinence period did not have a negative effect on sperm quality, as represented by sperm motility and SDF. The IVF cycle for oligozoospermic men can be improved by consecutive semen collection within 4 hours in addition to the first semen sample in some circumstances.
Supplementary material
Supplementary material can be found via https://doi.org/10.5653/cerm.2023.06100.
(A) Non-clumped cells are gated. (B) Cells containing fragmented DNA were measured from the single cells by propidium iodide (PI)-area positive and fluorescein (FITC) positive (Q2). (C) Negative control without adding terminal deoxynucleotidyl transferase enzyme.
Sperm DNA fragmentation from terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assays are shown. Number 1 to 34 represent the patients. Panels (A) on the left side represents 2 to 7 days abstinent semen. Panels (B) on the right side represents short abstinent semen. Cells containing fragmented DNA were measured from the single cells by propidium iodide (PI)-area positive and fluorescein (FITC) positive on the right upper quadrants of the each graphs. UL, left upper quadrant; UR, right upper quadrant; LL, left lower quadrant; LR, right lower quadrant.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Author contributions
Conceptualization: NP, AS. Data curation: NP, AT, SC, CS, AS. Formal analysis: NP, CS, AS. Methodology: AT, SC. Project administration: NP, AT, SC. Writing-original draft: NP, AS. Writing-review & editing: AS.
Acknowledgements
We thank the entire Ramathibodi IVF Laboratory staff for their technical assistance.