Clinical and laboratory factors associated with the presence of dysmorphic oocytes in intracytoplasmic sperm injection cycles
Article information
Abstract
Objective
This study investigated the clinical and laboratory factors associated with the presence of dysmorphic oocytes in intracytoplasmic sperm injection (ICSI) cycles.
Methods
The study involved 200 ICSI cycles, performed from 2020 to 2021, that yielded at least one mature oocyte. Clinical characteristics and ovarian stimulation methods were compared between 68 cycles with at least one dysmorphic oocyte (the dysmorphic group) and 132 cycles with normal-form oocytes only (the non-dysmorphic group). Dysmorphic oocytes were characterized by dark cytoplasm, cytoplasmic granularity, cytoplasmic vacuoles, refractile bodies in the cytoplasm, smooth endoplasmic reticulum in the cytoplasm, an oval shape, an abnormal zona pellucida, a large perivitelline space, debris in the perivitelline space, or an abnormal polar body.
Results
The ages of the women, indications for in vitro fertilization, serum anti-Müllerian hormone levels, and rates of current ovarian endometrioma were similar between the dysmorphic and non-dysmorphic groups. In both groups, the three ovarian stimulation regimens, two types of pituitary suppression, and total gonadotropin dose were employed similarly. However, the dual-trigger method was used more frequently in the dysmorphic group (67.6% vs. 50%, p=0.024). The dysmorphic group contained significantly more immature oocytes and exhibited significantly lower oocyte maturity (50% vs. 66.7%, p=0.001) than the non-dysmorphic cycles. Within the dysmorphic group, significantly lower oocyte maturity was found in the cycles using a dual-trigger, but not in those with a human chorionic gonadotropin trigger.
Conclusion
ICSI cycles with dysmorphic oocytes are closely associated with reduced oocyte maturity. This association was observed exclusively in dual-trigger cycles.
Introduction
Obtaining good-quality metaphase II oocytes is an essential prerequisite for human in vitro fertilization (IVF) programs. Several criteria are used to determine the quality of a mature oocyte. These include the compactness and thickness of the cumulus-oocyte complex; the brightness of the cytoplasm; the granularity and clustering of organelles within the cytoplasm; the polar body (PB) shape, size, and appearance; the thickness and structure of the zona pellucida (ZP); the size and granulation of the perivitelline space (PVS); and the location and refraction of meiotic spindles [1,2]. Typically, a mature oocyte with clear or moderately granular cytoplasm, a narrow PVS, a normal PB shape, and a colorless and birefringent ZP is considered to be of good-quality [3].
Dysmorphic oocytes can be categorized based on various characteristics, including a dark cytoplasm, cytoplasmic granularity, cytoplasmic vacuoles, refractile bodies in the cytoplasm, the presence of smooth endoplasmic reticulum (SER) in the cytoplasm, an oval shape, an abnormal ZP, a large PVS, debris in the PVS, or an abnormal PB [4]. While some oocytes display only one type of dysmorphism, others may present with two or more abnormalities.
In our previous report, we noted a 58% (58 of 100) incidence of dysmorphic oocytes in 35 intracytoplasmic sperm injection (ICSI) cycles, each of which yielded at least one dysmorphic oocyte. However, when considering all 154 ICSI cycles, including those that produced only oocytes of normal-form, the incidence rate dropped to 10.7% (58 of 541) [4].
Numerous studies have revealed that fertilization and embryonic development are comparable between dysmorphic and normal-form oocytes [2,5,6]. Separate research has indicated that the morphology of the first PB does not negatively impact embryo development [7,8].
However, in other studies, oocytes with dysmorphic characteristics have been found to exhibit a lower fertilization rate compared to normal-form oocytes [9-12]. In a study by Rienzi et al. [12], the presence of vacuoles, an abnormal first PB, and a large PVS were associated with a decreased fertilization rate. Regarding embryo development and quality, the presence of SER clusters, a large PVS, and shape abnormalities are considered poor prognostic factors [13].
We previously reported that dysmorphic oocytes exhibited a significantly lower fertilization and cleavage rate, even with ICSI applied. However, these oocytes demonstrated a comparable rate of producing top- or good-quality embryos to that of normal-form oocytes [4]. Our report also indicated that oocytes with dark cytoplasm, abnormal PBs, or cytoplasmic vacuoles had a favorable prognosis, as evidenced by the percentage of top-quality embryos produced [4].
The origin of morphological abnormalities in oocytes remains largely unknown, but it is likely multifactorial. Intrinsic factors, such as age and genetic defects, as well as extrinsic factors, such as the ovarian stimulation protocol or handling procedures following oocyte retrieval, have been suggested [14].
Several studies have been conducted on the conditions associated with higher retrieval of dysmorphic oocytes. In one prospective study, the rate of dysmorphic oocytes was found to be similar between a group with two or fewer immature oocytes and a group with more than two immature oocytes. However, a wider PVS was more commonly observed in the group with two or fewer immature oocytes [15].
A retrospective study revealed that the serum anti-Müllerian hormone (AMH) level was inversely associated with cytoplasm granulation, abnormally amorphous oocytes, extended PVS, granulated PVS, fragmented PB, and oocyte morphology score as represented by the average oocyte quality index (AOQI) [16]. The AOQI was established by Sigala et al. [17] in 2015. This index is calculated by counting the number of abnormalities in oocyte morphology across seven categories: cytoplasmic granularity, irregular shape or thickened ZP, presence of intracytoplasmic vacuoles, materials in the PVS, anomalies of the first PB, large PVS, and oocyte shape. The index is then calculated as the ratio of the total number of abnormalities to the number of metaphase II oocytes [17].
In another retrospective study, the AOQI was found to be similar between women with and without endometriosis. However, two specific abnormalities—abnormal oocyte shape and intracytoplasmic vacuoles—were observed more frequently in women with endometriosis [18].
The objective of this study was to examine whether the presence of dysmorphic oocytes in ICSI cycles is associated with various clinical and laboratory factors. The clinical factors considered included the age of the woman, serum AMH level, diagnosis of endometriosis, dose or type of gonadotropin used, pituitary suppression methods, and triggering agents. The laboratory factors considered include the number and maturity of oocytes.
Methods
1. Study participants
We conducted a retrospective review of data from 200 ICSI cycles, involving 121 women, carried out at Seoul National University Bundang Hospital between 2020 and 2022. The selection criteria included: (1) the retrieval of at least one mature oocyte; (2) the use of recombinant follicle-stimulating hormone (FSH), urinary human menopausal gonadotropin, or a combination of both as the ovarian stimulation agent (excluding follitropin delta, mild stimulation, a combination of gonadotropins and oral agents, or a natural cycle); (3) the use of a gonadotropin-releasing hormone (GnRH) agonist or a GnRH antagonist for pituitary suppression; and (4) the use of human chorionic gonadotropin (hCG) or a combination of hCG and a GnRH agonist (that is, a dual method) for the final trigger. The Institutional Review Board of Seoul National University Bundang Hospital (B-2302-808-102) granted approval for this study. Written informed consent by the patients was waived due to a retrospective nature of our study.
An oocyte was classified as dysmorphic if it exhibited any of the following characteristics: dark cytoplasm, cytoplasmic granularity, cytoplasmic vacuoles, refractile bodies within the cytoplasm, SER in the cytoplasm, an oval shape, an abnormal ZP, a large PVS, debris within the PVS, or an abnormal PB. This classification is consistent with our previous report [4].
In 68 ICSI cycles, at least one dysmorphic oocyte was obtained; this was considered the dysmorphic group. In contrast, in 132 cycles, no dysmorphic oocytes were found (that is, only normal-form oocytes were present; this was considered the non-dysmorphic group). Data regarding each woman, including age, body mass index, indications for IVF, endometriosis diagnosis, current presence of ovarian endometrioma, and serum AMH level, were gathered via chart review. Serum AMH levels were measured using fully automated AMH assays (Beckman Coulter or Roche Diagnostics).
2. Ovarian stimulation and oocyte retrieval
Ovarian stimulation was performed using one of the following regimens: recombinant FSH (Gonal-f, Merck-Serono; or GONADOPIN-NF, Donga-ST); highly purified urinary FSH (IVF-M; LG Chem); recombinant FSH in combination with any urinary gonadotropins (IVF-M or Menopur; Ferring).
Cycles stimulated with follitropin delta, recombinant FSH in conjunction with recombinant luteinizing hormone (Pergoveris; Merck-Serono), mild stimulation, any combination of gonadotropins, and any oral agents (including aromatase inhibitors or clomiphene citrate) were excluded. Cycles stimulated in combination with growth hormone were also excluded. Pituitary suppression was achieved using either a daily GnRH agonist long protocol (Decapeptyl; Ferring) or a flexible daily GnRH antagonist protocol (Cetrotide, Merck-Serono; or Ganirelix, LG Chem).
When the leading follicle reached a diameter of 18 to 19 mm, a final trigger was administered using either 250 μg of recombinant hCG (Ovidrel; Merck-Serono) or a combination of recombinant hCG and GnRH agonist (Decapeptyl 0.2 mg), also known as dual triggering. Oocytes were then retrieved 35 to 36 hours later. The oocytes were denuded using 85 IU/mL hyaluronidase (Cook) and mechanical pipetting. An oocyte was defined as mature if the first PB was present and the germinal vesicle was absent. In contrast, an oocyte was considered immature if it either contained a germinal vesicle or lacked both a germinal vesicle and the first PB.
3. Data analysis
Statistical analysis was performed using SPSS version 26.0 (IBM Corp). All variables were presented as the median (interquartile range), and the Mann-Whitney U test was employed to compare medians. The Pearson chi-square test or the Fisher exact test was used to compare proportions. A p-value of less than 0.05 was considered to indicate statistical significance.
Results
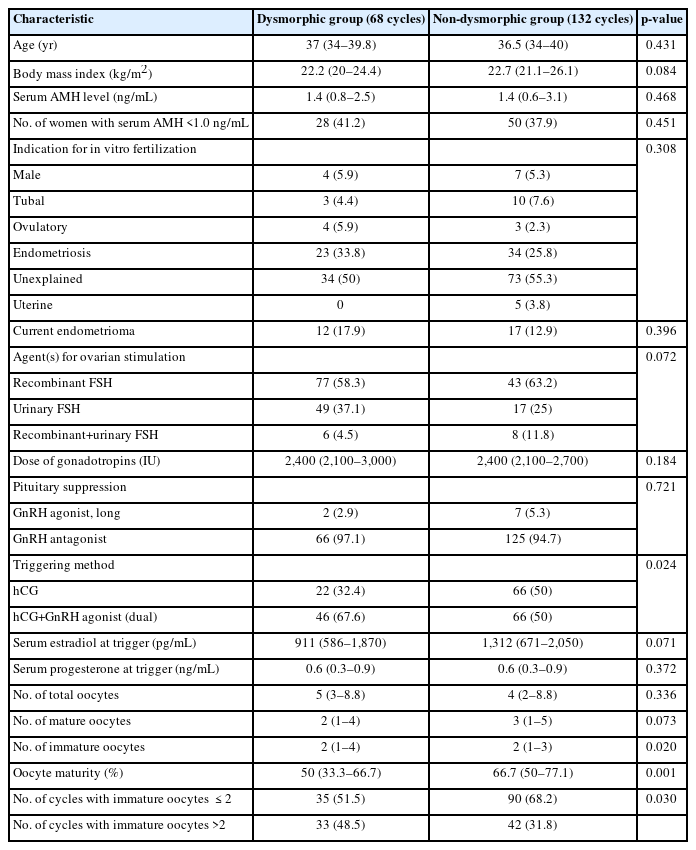
Age, body mass index, indications for IVF, serum AMH levels, and the proportions of participants with serum AMH ≤1.0 ng/mL were similar between the dysmorphic and non-dysmorphic groups (Table 1). The proportions of endometriosis as an indication for IVF and the current presence of ovarian endometrioma were also similar between groups.
In both groups, the three types of ovarian stimulation agents (recombinant FSH, highly purified urinary FSH, and recombinant FSH in combination with any urinary gonadotropins) were used similarly. The total dose of gonadotropins was also comparable between groups.
The use of a GnRH antagonist for pituitary suppression was predominant in both groups (97.1% for the dysmorphic group vs. 94.7% for the non-dysmorphic group). However, the dual-trigger method was more frequently used in the dysmorphic group (67.6% vs. 50%, p=0.024).
While the total numbers of oocytes and mature oocytes were similar between the two groups, the dysmorphic group had a significantly higher number of immature oocytes, resulting in a significantly lower oocyte maturity rate (50% vs. 66.7%, p=0.001). In fact, the percentage of cycles with more than two immature oocytes was significantly higher in the dysmorphic group (48.5% vs. 31.8%, p=0.030).
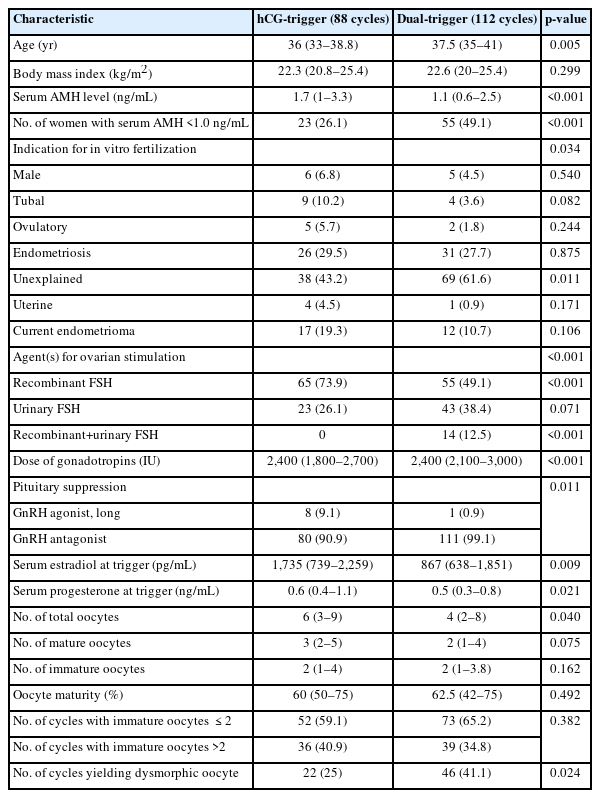
Because the dual-trigger method was more frequently used in the dysmorphic group, we compared various clinical characteristics and ovarian stimulation outcomes between the cycles with an hCG-trigger (88 cycles) and those with a dual-trigger (112 cycles). As illustrated in Table 2, the dual-trigger group exhibited characteristics consistent with diminished ovarian reserve and/or poor ovarian response. Specifically, the women in this group were older and had lower serum AMH levels relative to the hCG-trigger group. Interestingly, despite higher gonadotropin usage in the dual-trigger group, both the serum estradiol level at trigger and the total number of oocytes were lower. Recombinant FSH was used less frequently in this group, while GnRH antagonist suppression was used more often. However, the number of immature oocytes was similar between the hCG-trigger and dual-trigger groups, as was the proportion of mature oocytes (60% vs. 62.5%, respectively). Notably, the proportion of cycles with dysmorphic oocytes present was significantly higher in the dual-trigger group (41.1% vs. 25%, p=0.024).
Given that the more frequent use of a dual-trigger in the dysmorphic group may act as a confounding factor, we compared the outcomes of ovarian stimulation between the dysmorphic and non-dysmorphic groups considering the trigger method used (Table 3). In the cycles with an hCG-trigger, the numbers of total, mature, and immature oocytes, as well as the oocyte maturity, were similar between the dysmorphic and non-dysmorphic groups. However, among the cycles with a dual-trigger, the dysmorphic group had a significantly higher number of immature oocytes and a significantly lower proportion of mature oocytes (50% vs. 66.7%, p<0.001). In fact, the percentage of cycles with more than two immature oocytes was significantly higher in the dysmorphic group (52.2% vs. 22.7%, p=0.002).
Discussion
In this study, the dysmorphic group exhibited a significantly higher number of immature oocytes and significantly lower oocyte maturity than the non-dysmorphic group. The proportion of cycles with more than two immature oocytes was also significantly higher in the dysmorphic group. These findings suggest that the overall inferior quality of the oocyte pool could impact the acquisition of dysmorphic oocytes.
The dual-trigger method was utilized more often in the dysmorphic group in the present study. As a result, our initial comparison focused on the various clinical characteristics and outcomes of ovarian stimulation between the hCG-trigger group (88 cycles) and the dual-trigger group (112 cycles). Subsequently, we compared the outcomes of ovarian stimulation between the dysmorphic and the non-dysmorphic groups by the trigger method.
We discovered that the dual-trigger group exhibited peculiar characteristics consistent with reduced ovarian reserve and/or poor ovarian response. This is believed to reflect the physician’s preference for a dual-trigger when conducting ovarian stimulation in women with diminished ovarian reserve or anticipated poor ovarian response. However, the quantity of immature oocytes and the level of oocyte maturity were comparable between the hCG-trigger and dual-trigger groups.
In a prior study, dual triggering resulted in a significantly higher number of mature oocytes and greater oocyte maturity than hCG-only triggering in young women with diminished ovarian reserve undergoing elective oocyte cryopreservation [19]. However, in previous research conducted by our team, we found that dual triggering yielded a similar number of oocytes and comparable oocyte maturity to hCG-only triggering in women with various malignancies or endometrioma who also underwent elective oocyte cryopreservation [20]. A systematic review and meta-analysis additionally reported similar numbers of oocytes and oocyte maturity proportions between hCG-only and dual triggers [21]. In the present study, we likewise found a similar number of immature oocytes and similar oocyte maturity between hCG-only and dual-trigger cycles. While the aim of this study was not to evaluate the effectiveness of dual triggering, our findings suggest that a dual-trigger is not associated with lower oocyte maturity.
However, the proportion of cycles featuring dysmorphic oocytes was significantly higher in the group that underwent dual triggering. Thus, the dual-trigger method could be a contributing factor in the acquisition of dysmorphic oocytes.
Furthermore, in the dysmorphic group, a significantly higher number of immature oocytes or lower oocyte maturity was found, but this was observed only in the dual-trigger group and not in the hCG-trigger group.
Collectively, we postulated that the dual-trigger method may contribute to a greater acquisition of dysmorphic oocytes. Furthermore, we found a close association between the presence of dysmorphic oocytes and lower oocyte maturity in ICSI cycles utilizing dual triggering.
In a prior study, the oocyte morphology score, represented by AOQI, was found to be comparable between women with and without endometriosis [18]. We also observed that the proportion of endometriosis as an indication for IVF, as well as the current presence of ovarian endometrioma, were similar between the dysmorphic and non-dysmorphic groups. Therefore, endometriosis may not be a considerable factor in the acquisition of dysmorphic oocytes.
A previous study indicated an inverse relationship between serum AMH level and oocyte morphology score, as represented by AOQI [16]. We did not evaluate the oocyte morphology score, so a direct comparison with our results was not possible. However, both the serum AMH levels and the proportion of women with diminished ovarian reserve were similar between the dysmorphic and non-dysmorphic groups. Therefore, it can be inferred that diminished ovarian reserve is not a contributing factor to the acquisition of dysmorphic oocytes.
In conclusion, we identified a close association between the presence of dysmorphic oocytes and lower oocyte maturity, particularly when dual triggering was used. Furthermore, this association between dysmorphic oocytes and lower oocyte maturity was observed only in ICSI cycles employing the dual-trigger method.
Notes
Conflict of interest
Byung Chul Jee has served as editor-in-chief of Clinical and Experimental Reproductive Medicine since 2018. However, he was not involved in the selection, evaluation, or decision-making process for the peer review of this article. No other potential conflicts of interest related to this article have been reported.
Author contributions
Conceptualization: TEK, BCJ. Data curation: TEK, HKL. Formal analysis: TEK, HKL, BCJ. Methodology: TEK, BCJ. Project administration: TEK, BCJ. Visualization: TEK. Writing-original draft: TEK, BCJ. Writing-review & editing: TEK, HKL, BCJ.