Review of potential spermatogenic and aphrodisiac effects of the Ferula genus
Article information
Abstract
Objective
Men’s sexual health plays an important role in male fertility and childbearing, as it is associated with factors such as sexual desire, healthy spermatogenesis, and erectile function. In various cultures, medicinal plants have been utilized to address male sexual issues, including infertility and erectile dysfunction. Despite recent advancements in medical science for treating male impotence, some men opt for herbal supplements as an alternative, given that numerous herbs have the potential to enhance male sexual performance. The Apiaceae family is one of the oldest plant families used for medicinal purposes. Ferula, a genus within this family, comprises approximately 170 different species worldwide. Members of this genus possess numerous therapeutic properties due to the presence of various compounds. This article aims to explore the potential impacts of Ferula plants on the male reproductive system.
Methods
This review article was prepared by searching for terms including Ferula and “aphrodisiac,” Ferula and “spermatogenesis,” and Ferula and “male reproductive system.” Relevant information was gathered through electronic databases, including ISI Web of Knowledge, PubMed, and Google Scholar.
Results
The findings indicated that relatively comprehensive studies have been conducted in this area, revealing that certain Ferula species have been employed in folk medicine to boost fertility and libido. Recent research has corroborated these effects.
Conclusion
It is hoped that new aphrodisiac compounds with fewer side effects can be isolated from Ferula plants in the future.
Introduction
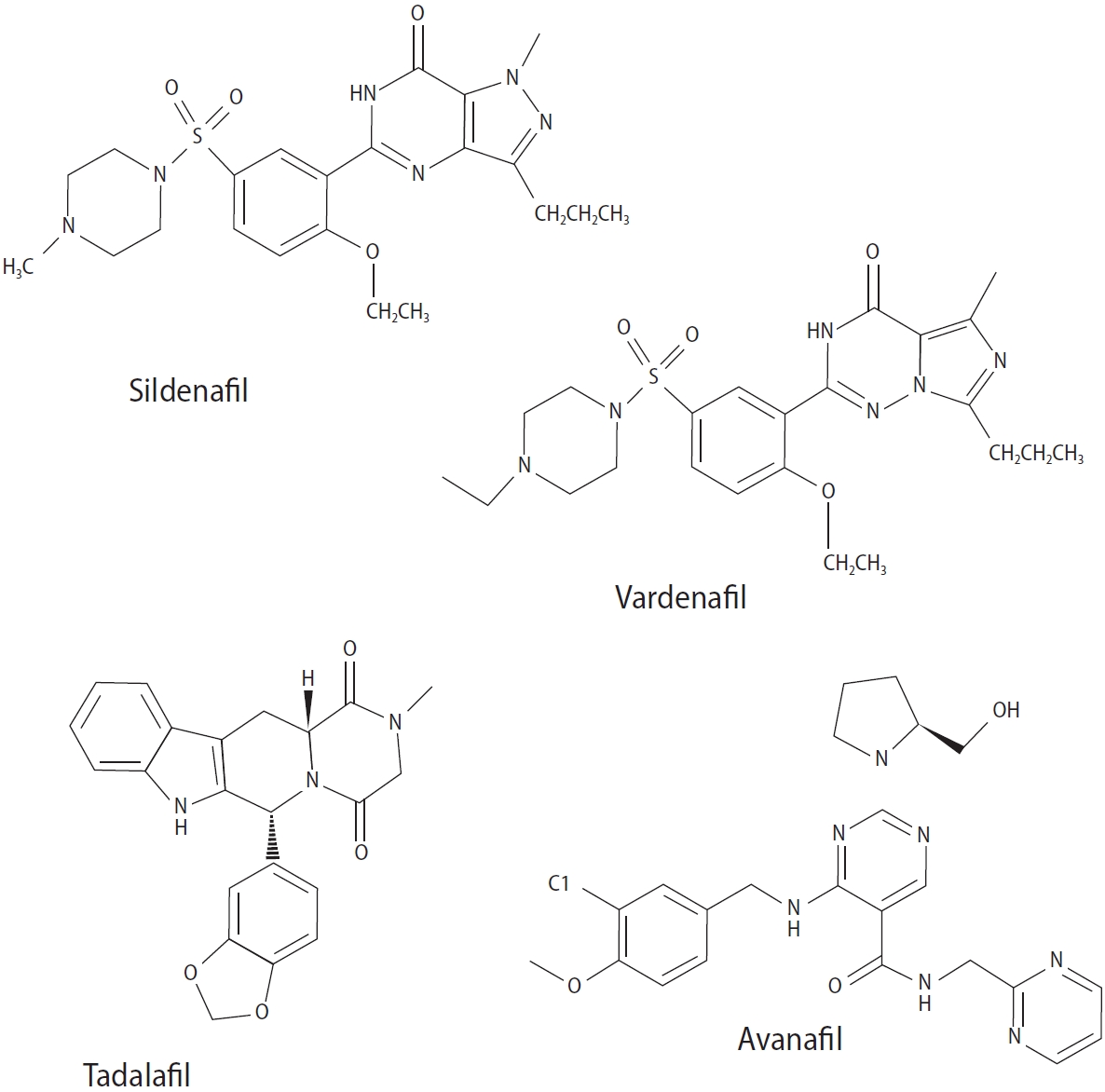
Erectile dysfunction (ED), loss of sexual interest, ejaculation disorders, and sperm abnormalities are key problems of the male reproductive system that play important roles in population growth and mental health [1]. Infertility in humans is characterized by the inability to conceive after 1 year of regular intercourse, affecting approximately 8% to 12% of couples worldwide. Male infertility accounts for roughly 40% to 50% of fertility failures [2]. Factors such as chemotherapy, antibiotics, radiation therapy, stress, pollution, poor eating habits, environmental factors, work, and lifestyle changes contribute to fertility issues and sperm abnormalities, which cause 30% to 40% of infertility cases [3]. Infertility treatments vary for several reasons and range from simple drug therapies to laboratory procedures and advanced surgery [4]. Primary testicular defects are a main cause of male infertility; these include abnormal sperm parameters such as number, morphology, and motility and account for 65% to 80% of cases [5]. In contrast, male sexual dysfunction refers to a collection of conditions, including ED and premature ejaculation (PE), which can ultimately result in male sexual dysfunction. ED is a globally prevalent disorder related to the male reproductive system that negatively impacts quality of life, particularly among older men [6]. This condition can be caused by factors such as androgen deficiency, atherosclerosis, diabetes, spinal cord injury, high cholesterol level, high blood pressure, prostate surgery, and psychological conditions such as depression [7]. Several medicinal compounds, known as phosphodiesterase type 5 inhibitors, are used to treat ED, including vardenafil, sildenafil, avanafil, and tadalafil (Figure 1) [8]. Separately, PE is defined as a lack of ejaculation control accompanied by distress, which can have physiological or psychological origins [9].
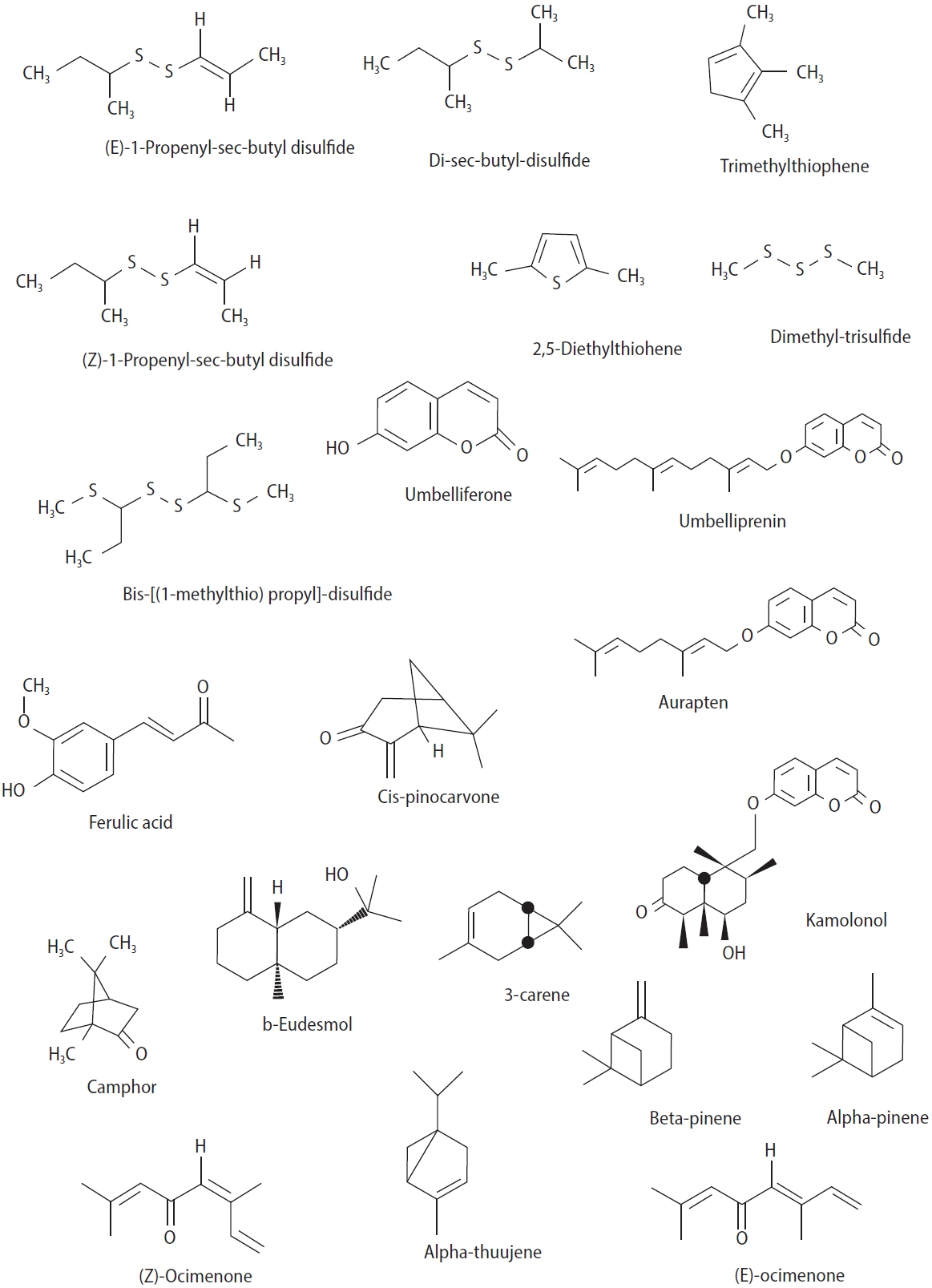
Generally, compounds used to increase sperm count or improve libido are associated with side effects. Complementary treatments and traditional medicine represent knowledge passed down through generations, based on local methods. Medicinal plants play an important role in the traditional medicine of various countries [10]. Apiaceae is a family of flowering plants that primarily grow in dry and temperate regions [11]. These plants typically have a pungent smell due to the presence of sulfide compounds and volatile essential oils [12]. The genus Ferula includes approximately 170 species that grow in areas ranging from Central Asia and the Mediterranean to North Africa [13]. These herbs have been reported in the scientific literature for their aphrodisiac and spermatogenic activity. Since ancient times, different species of Ferula have been used as aphrodisiacs in traditional and folk medicine across countries. In traditional Turkish medicine, several species of Ferula, such as the root and oleo gum resin of Ferula elaeochytris, Ferula communis, Ferula assa-foetida, and Ferula gummosa, have been used as aphrodisiacs to treat male sexual disorders. Ferula hermonis, also known as “zallouh” in the Middle East, is still used as a male aphrodisiac [14]. In Iranian traditional medicine, F. assa-foetida has also been used as an aphrodisiac [15]. In the Ayurvedic system of ancient India as well as in South American traditional medicine, such as in Brazil, asafoetida is considered an aphrodisiac [16]. In Nepal, asafoetida is used daily, primarily as an aphrodisiac for men [17]. Ibn Sina (Avicenna) and Al-Antaki also emphasized the aphrodisiac effects of F. assa-foetida [18]. Scientific investigations have demonstrated a number of medicinal properties of Ferula, including antinociceptive [19], antihemolytic and antioxidant [20], anticoagulant [21], anticonvulsant [22], relaxant [23], memory enhancement [24,25], antihyperglycemic [26], acetylcholinesterase inhibitory [27], antidepressant [28], antiulcer [15], antitumor [29], and anti-demyelination [30] properties. A phytochemical analysis of plants in this genus has shown that they contain various compounds, including terpenoids, coumarins, and many sulfide compounds (Figure 2) [31]. Due to the widespread use of these plants in the treatment and prevention of male sexual dysfunction, this review article is focused on scientific evidence of the effects of various compounds and extracts of these plants on increasing spermatogenesis and aphrodisiac properties.
Methods
Articles were retrieved using various combinations of keywords, such as “male reproductive system,” “Ferula,” “aphrodisiac,” and “spermatogenesis,” from scientific databases such as PubMed, Google Scholar, and Science Direct. Studies published through the end of 2022 that focused on the male reproductive system and extracts or compounds isolated from the genus Ferula were summarized and included in this paper (Table 1). The primary limitations of this study were the inability to access the full texts of some articles and the lack of subscriptions to certain journals by our academic center.
Ferula hermonis
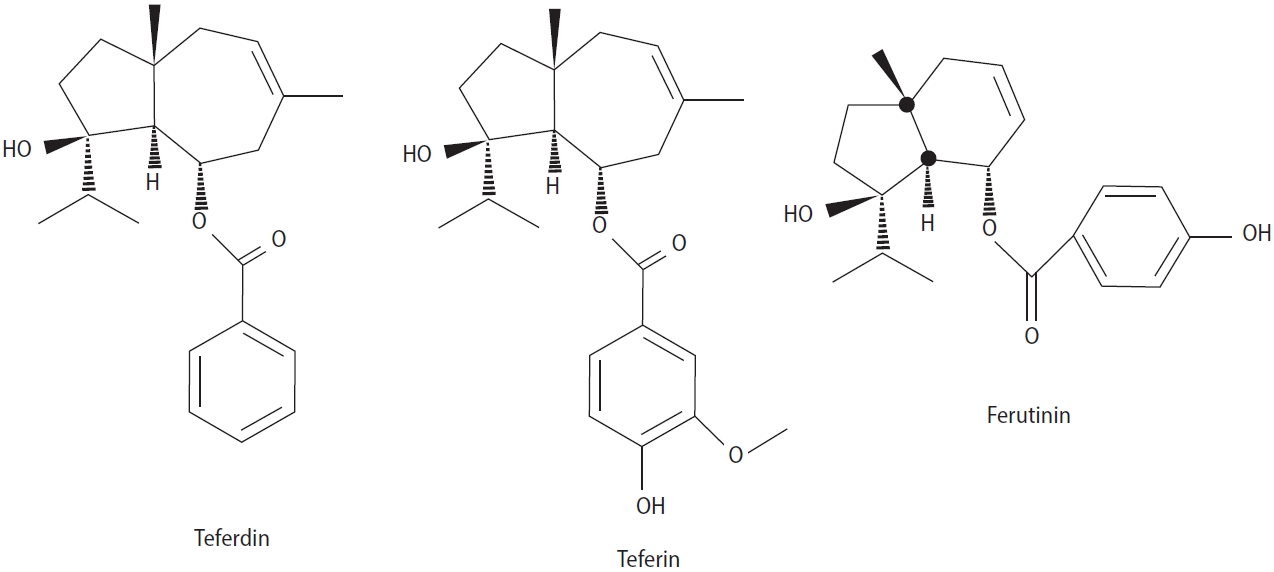
F. hermonis Boiss is a perennial plant found in limited geographical areas, including Lebanon and Syria. Among the various Ferula species evaluated, this species has been most extensively studied for its aphrodisiac effects and capacity to improve sperm health. In different cultures and nationalities, this plant is used to increase libido. The plant’s root is commonly described as “Lebanese root” or “Lebanese viagra.” It has been used in folk medicine to reduce total body weight and plasma cholesterol levels, as well as to treat impotence and frostbite, skin infections, stomach disorders, fever, dysentery, and neurological disorders such as hysteria [32]. Thermal stress can adversely affect physiology in various ways, such as reducing fertility, activity, respiration rate, and food intake. A study examining the use of F. hermonis water root extract (0.025 mL/100 g for 8 weeks, orally) demonstrated that the extract can prevent the harmful effects of heat stress on sperm number, motility, morphology, and viability while significantly increasing testosterone level [33]. Examining the effects of 100 and 200 mg/kg of F. hermonis root extract in ducks revealed that the relative weight, length, width, and volume of the testicles, the diameter of the seminiferous tubules, and the thickness of the seminiferous epithelium increased. Additionally, luteinizing hormone (LH), follicle-stimulating hormone (FSH), and testosterone levels significantly increased [34]. One study showed that F. hermonis alcoholic root extract (0.03 mL/50 mL) can reduce sperm abnormalities in Holstein bulls caused by freezing with different storage periods (2, 30, and 60 days after freezing) [35]. This study demonstrates that the plant extract has protective effects on sperm against freezing. A study on the effect of 600 mg/kg of oral F. hermonis root extracts (petroleum ether, ethyl acetate, methanol, and water) on the sexual behavior of male rats showed that the petroleum ether and ethyl acetate extracts significantly decreased mount rate, intromission rate, and intromission latency. The methanolic extract caused a significant increase in mount rate, while the water extract impacted only intromission latency [36]. The protective effects of F. hermonis are not limited to that study. Research has shown that F. hermonis not only increases testicular function and sexual desire but also can play a protective role against drugs that damage the testicles and the process of spermatogenesis. For example, Cycram is used in neoplastic treatment, but this medication also has destructive effects on testicular tissue. A related study showed that the simultaneous use of the methanol extract of F. hermonis root at a dose of 0.025/100 g improved sperm indices and testosterone levels, which apparently related to the reduction of plasma lipid peroxidation levels and the increase of antioxidant enzyme activity in the tested animals [37]. Furthermore, the simultaneous use of F. hermonis extract (3 mg/kg) and bee honey (1.5 mL/kg) for 10 consecutive days showed that this extract had a protective effect on the testis against gamma radiation (8 Gy). The weight of the testis and antioxidant indices improved, and the serum levels of testosterone, FSH, and LH were restored [38]. Contrary to these results, other studies have indicated that F. hermonis has no significant effect on the improvement of sperm or sexual behavior and may even cause damage and deterioration of sperm indicators. Administration of an aqueous extract of F. hermonis to male rats (3 mg/kg) for 6 weeks caused a decrease in the weight of the accessory sexual organs. Additionally, the number of epididymal sperm and their motility significantly decreased, and sperm abnormalities significantly increased [39]. In another study, oral administration of aqueous extract of F. hermonis root (6 mg/kg) to mice for 6 weeks significantly decreased testosterone levels and caused fertility impairment. Histopathological degenerative changes and a significant decrease in the expression of estrogen receptor b in the testes, epididymis, and seminal vesicles were also observed [40]. Consumption of 1.5, 3, and 6 mg/kg of the aqueous extract of F. hermonis root for 3 and 6 weeks was associated with decreased testosterone levels and severe histopathological damage in the testis [41]. This issue may be related to the duration of use, the type of extraction, and the concentration used. Studies have shown that long-term use of F. hermonis can also cause toxicity and testicular atrophy. El-Thaher et al. [42] showed that administration of the essential oil of F. hermonis seeds improved the penile erection index in a dose-dependent manner, but its use also caused toxicity after 28 days. According to some findings, F. hermonis can strongly impact sexual motivation only acutely, and it does not have a significant effect when consumed sub-chronically. Zanoli et al. [43] found that the injection of 30 and 60 mg/kg of F. hermonis extract increased the sexual motivation of normal animals and led to improvements among sluggish/impotent male rats, but long-term use did not have a significant effect. These results showed that F. hermonis can exert dual and opposite effects. Such impacts are related to the increase in testosterone levels, as the injection of 0.01 and 0.02 mL/100 g/body weight of 50% aqueous extract of F. hermonis has been shown to increase the testosterone level in male rats [44]. However, limited studies have been conducted on the effective compounds of this plant. Ferutinin, isolated from F. hermonis, is an oxygenated sesquiterpene that appears to act through the nitric oxide signaling pathway [45]. The injection of the three compounds of ferutinin, teferdin, and teferin (Figure 3) isolated from F. hermonis in sexually potent and sluggish/impotent animals with an acute (2.5 mg/kg) and sub-chronic (0.25 mg/kg per day for 10 days) regimen showed that ferutinin and teferdin can reduce mounting and intromission latencies and shorten ejaculation latency only in the acute regimen both compounds increased testosterone levels in rats, but only teferdin increased sex appetite and testosterone levels sub-chronically, while sub-chronic ferutinin had a negative effect and decreased testosterone levels with long-term use [46].
Ferula assa-foetida
F. assa-foetida is a native plant of Iran and Afghanistan that naturally grows in the hot and dry central regions of Iran. This plant is one of the most well-known species of Ferula and has been used to treat various diseases [47]. It is a perennial plant, approximately 2 m in length, with a strong and unpleasant odor. A sticky gum, called anghuzeh in Persian and “asafoetida” in scientific terms, is obtained from cutting its roots [30]. The main components of asafoetida generally include sulfide, glycosidic, and various sesquiterpene coumarin compounds [48]. In many countries, such as Iran, Afghanistan, Nepal, and Arabic countries, this plant is used as an aphrodisiac [49]. In Iranian traditional medicine, it has also been mentioned as an aphrodisiac, and pregnant women are advised to avoid consuming it due to the risk of miscarriage [50]. Feeding Karakul rams a diet of 6 kg of compound feed and 3 kg of F. assa-foetida seeds for 30 days resulted in a 27.3% increase in ejaculation capacity, a 20% increase in sperm concentration, an 80% increase in the number of live sperm, and a 19.6% increase in sperm motility. Additionally, sperm breathing increased significantly (114.3%) within 15 minutes [51]. Apparently, although asafoetida increases sperm count and improves its quality, it may cause a decrease in plasma testosterone concentration, especially at high doses. In one study, injecting 50 mg/kg of asafoetida aqueous extract resulted in a decreased plasma testosterone concentration in the treatment group, but sperm count and quality were increased significantly [52]. In separate research, administering 75, 150, and 300 mg of asafoetida extract to rats for 15 days led to a decrease in the thickness of the cell layers of the seminiferous tubules, and the number of Leydig and Sertoli cells, as well as blood testosterone concentration, decreased significantly compared to the control group [53]. Injecting asafoetida at doses of 25, 50, 100, and 200 mg/kg into male rats for 6 weeks showed that asafoetida can increase sperm motility and improve sperm morphology. However, at a high dose (200 mg/kg), Leydig cells became vacuolated, and testosterone levels decreased [54]. Although the positive effect of asafoetida on spermatogenesis has been determined in various studies, sufficient consideration should be given to the dose and duration of its use. These results suggest that additional studies are needed to clarify the toxicity of this plant. One study on this topic showed that long-term use with a dose of 200 mg/kg and above can increase liver enzymes and potentially cause liver damage [55]. In addition to the gum, the seeds of F. assa-foetida also appear to have aphrodisiac and spermatogenic effects. In rats, oral consumption of hydroalcoholic extract from the seeds of F. assa-foetida for 6 weeks at a dose of 50 mg/kg significantly improved fertility parameters, including sperm count, sperm morphology, and motility, and increased testosterone and LH levels in the treated group [56]. “Masculinity” tablets, prepared from seeds and 50% water-ethanol extracts from the roots of F. assa-foetida, were found to increase erection in rats and humans. This combination also led to a quantitative and qualitative improvement in sperm count for individuals with untreatable azoospermia and incomplete azoospermia after 2 months of treatment, by 17% and 60%, respectively. A significant improvement in libido and erectile function in men was also reported [57].
Ferula huber
Ferula huber is one of several species of the genus Ferula, native to Turkey and widely used in the traditional medicine of that country. Yusufoglu et al. [58] demonstrated that the methanol extract of F. huber has significant antioxidant and hypoglycemic activities. Like many other Ferula species, F. huber is utilized as an aphrodisiac in traditional Turkish medicine. The effects of aqueous and chloroform extracts from the root of the F. huber plant, as well as gummosin, mogoltavidin, deacetylkellerin, ferukrin acetate with kellerin, elaeochytrin-A, and ferutinin isolated from the chloroform extract, were tested on the aphrodisiac potential of rats. The results showed that only the chloroform extract and three compounds—deacetylkellerin, elaeochytrin-A, and ferutinin (Figure 4)—had a significant impact on stimulating sexual behaviors. Among these sesquiterpenoids, ferutinin was shown be the most effective aphrodisiac compound [14].
Ferula orientalis
Ferula orientalis, known as heliz in Turkey, is used as an antiseptic, sedative, antispasmodic, laxative, digestive, expectorant, diuretic, aphrodisiac, and stimulant [59]. The application of root extract from this plant has been shown to induce 98% relaxation in corpus cavernosum strips in response to acetylcholine (1 mM), electrical field stimulation (10 Hz), and sodium nitroprusside (0.1 μM), thereby enhancing erectile function in diabetic animals [60].
Ferula elaeochytris
F. elaeochytris, a perennial herb native to Turkey and known as çakşır in Anatolia, is widely used for its aphrodisiac, antioxidant, anti-inflammatory, and anti-diabetic properties. ED is an important complication of diabetes, leading to impotence in men with the condition. One study demonstrated that the methanol root extract of F. elaeochytris, at concentrations of 40 and 60 mg/kg, improved sexual parameters such as intracavernosal pressure (ICP) and testicular weight after 8 weeks, while also reducing blood sugar levels. Histopathological results indicated that extract consumption significantly increased spermatogenesis, the ICP/mean arterial pressure (MAP) ratio, and testis weight in diabetic animals [61]. Another study found that using a 1% concentration of F. elaeochytri extract improved sperm parameters in adult male redfish (Carassius auratus) after 60 days, whereas a 0.5% dose decreased sperm parameters and increased oxidative stress [62]. Thus, it is crucial to consider the appropriate concentration of medicinal plants before use. ED is an age-related side effect that can diminish quality of life among older individuals. Consequently, discovering new compounds to alleviate this disorder can greatly enhance the lives of the elderly. A study involving the oral administration of F. elaeochytris extract at concentrations of 40 and 20 mg/kg in aged rats revealed that the extract could positively impact changes in smooth muscle cells and collagen fibrils, tumor necrosis factor-α level, penile neuronal nitric oxide synthase expression, serum testosterone level, neurogenic and endothelial-dependent relaxation of the corpus cavernosum, the ICP/MAP ratio, total antioxidant status, and total oxidant status in corpus cavernosal tissue. The extract also increased serum testosterone levels, decreased tumor necrosis factor-α levels, and balanced oxidative and antioxidant parameters [63].
Ferula drudeana
Ferula drudeana is an indigenous plant found in the central regions of Turkey, traditionally used as an aphrodisiac. The primary components of the fruit’s essential oil are shyobunone (44.2%) and 6-epishyobunone (12.6%), and it has been established that various parts of the plant, such as the root and fruit, possess antibacterial properties [64]. Additionally, another study has demonstrated anti-diabetic and antioxidant effects [58]. A CHCl3 solution fraction (200 mg/kg body weight) and three compounds isolated from this plant, namely feselol, samarcandin, and 3′-O-acetyl samarcandin (Figure 5), were found to significantly increase mount frequency, intromission frequency, ejaculation latency, and postejaculatory interval in male rats. The mount and intromission latencies were notably reduced, while ejaculation latency was extended. Both feselol and samarcandin contributed to the decrease in postejaculatory interval, with samarcandin exhibiting a more potent aphrodisiac effect than feselol [65].
Ferula communis
F. communis is a Mediterranean umbelliferous plant with a rich history of medicinal and ecologically interesting uses. It naturally grows in the barren lands of the eastern Mediterranean mountainous regions of Turkey [66]. Some individuals consume the ground root of F. communis mixed with honey to boost libido. Goat herders and indigenous peoples assert that this plant has a sexually enhancing effect on both animals and humans. Additionally, F. communis is traditionally used as an anti-hysteria treatment and for treating dysentery. Research on F. communis has revealed that this plant contains two main types of phytochemicals: sesquiterpene daucane esters and prenylated coumarins [67]. A study involving the administration of a food supplement containing 0.2% F. communis extract for 90 days in fish demonstrated that the seminiferous lobules in the treatment group were larger and contained considerably more sperm, while no degenerative changes were observed. These findings suggest that this extract improves reproductive performance and may be effective in increasing ovarian and testicular capacity [68].
Conclusion
Findings from studies on the aphrodisiac and spermatogenic effects of the Ferula genus indicate that these plants hold promise for further research. It is well-known that these plants have been utilized as aphrodisiacs in the Middle East since ancient times. The recent results largely support the claims made by traditional medicine in this area. Among the plants in this genus, F. hermonis stands out as particularly noteworthy, and it appears that ferutinin, one of the isolated compounds, has a more potent effect on the male reproductive system than other compounds. These discoveries provide valuable insights for researchers to build upon in future studies. Such studies should focus on isolating additional compounds, determining the toxicity of these isolated compounds, and ultimately conducting human and clinical trials.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Author contributions
Conceptualization: SMB, NA. Data curation: AS, JAG, MY, NA. Formal analysis: MY. Funding acquisition: AS. Methodology: SMB. Project administration: SMB. Visualization: AS. Writing-original draft: SMB, AS. Writing-review & editing: MY.
Acknowledgements
We are very grateful to the staff of the Yazd Neuroendocrinology Research Center. We also very thankful from Dr. Fatemeh Zare Mehrjardi for facilitating the project.