The effect of various assisted hatching techniques on the mouse early embryo development
Article information
Abstract
Objective
In search of an ideal method of assisted hatching (AH), we compared the effects of conventional micropipette-AH and laser-AH on the blastocyst formation rate (BFR) and blastocyst cell numbers.
Methods
Four- to five-week-old ICR female mice were paired with male mice after superovulation using Pregnant mare's serum gonadotropin (PMSG) and hCG. The two-cell embryos were flushed from the oviducts of female mice. The retrieved two-cell embryos underwent one of five AH procedures: single mechanical assisted hatching (sMAH); cross mechanical assisted hatching (cMAH); single laser assisted hatching (sLAH); quarter laser assisted hatching (qLAH); and quarter laser zona thinning assisted hatching (qLZT-AH). After 72 hours incubation, double immunofluorescence staining was performed.
Results
Following a 72 hours incubation, a higher hatching BFR was observed in the control, sMAH, cMAH, and sLAH groups, compared to those in the qLAH and qLZT-AH groups (p<0.05). The hatched BFR was significantly higher in the qLAH and qLZT-AH groups than in the others (p<0.05 for each group). The inner cell mass (ICM) was higher in the control and sMAH group (p<0.05). The trophectoderm cell number was higher in the cMAH and qLAH groups (p<0.05).
Conclusion
Our results showed that the hatched BFR was higher in groups exposed the the qLAH and qLZT-AH methods compared to groups exposed to other AH methods. In the qLAH group, although the total cell number was significantly higher than in controls, the ICM ratio was significantly lower in than controls.
Introduction
The introduction of micromanipulation techniques was a landmark in the advancement of human assisted reproduction technology (ART).
First, the ICSI technique resolved numerous problems related to male factor infertilities [1,2,3]. Secondly, the pre-implantation genetic diagnosis (PGD) technique enabled safer pregnancies in couples at risk of hereditary genetic disorders [4]. Thirdly, assisted hatching (AH) techniques greatly increased the embryo implantation rate [4,5].
The zona pellucida (ZP) surrounding the oocyte is an extracellular matrix that prohibits polyspermy [6], supports embryonic development, prevents adhesion of the embryo on the oviduct [7], helps transport the embryo into the uterus, and protects against bacterial or fungal infection [8]. An oocyte after being fertilized, continues its cell division while protected by the ZP, and reachs the uterine cavity in either the morula or blastocyst stage. Hatching, the emergence of the blastocyst from the ZP, is essential for the implantation process.
AH is a procedure that helps facilitate the physiological hatching process by manipulating the ZP. The first human embryo AH method reported in the early 1990s used a sharp micropipette to dissect the ZP [5,9,10]. Subsequently, AH with acidic Tyrode's solution (pH 2.4) for zona drilling or partial thinning was reported [11], and this method is now widely used to improve both implantation and pregnancy rates [12,13,14]. This chemical AH is a simple procedure, requiring only a brief exposure to the acidic solution, which creates a hole or produces a thinning in the ZP. Furthermore, pre-existing micromanipulators can be used to hold the micropipettes, and the process can be readily visualized under a light microscope. The visualization allows users to easily identify the exact point of desired dissection on the ZP in a safe manner, as is popular in many laboratories [15,16,17]. However, chemical AH can result in embryo damage if the acid solution leaks into the ZP, and AH using micropipettes contains a possible risk of stabbing the blastomeres, resulting in cell death. Both methods rely heavily on the skills of the operator, and the size and extent of dissection or thinning are difficult to standardize. Potentially harmful effects on embryo development of the exposure of unfertilized oocyte ZP to chemicals have been reported [11]. Toxicity to the embryo is another unrevealed risk following exposure to some chemical solutions [18].
Recently, a laser AH method (LAH) was introduced to overcome the shortcomings of other methods [19]. LAH produces a hole on the ZP with a laser beam, which is relatively easy and does not require additional expendables or reagents. In addition, the size of the laser beam can be adjusted according to the ZP thickness, and a single laser beam emission is sufficient to complete the AH process. Through this method, the dissection of ZP can be standardized or customized according to the size of the ZP. More recently, it has been reported that the laser can be programmed to follow a virtual line drawn on a monitoring screen, making it possible to drill a larger hole or produce an even thinning of the ZP [20,21].
In order to determine the most appropriate AH method for human IVF, blastocyst formation rate (BFR) was examined following various AH methods in two-cell embryos. After a 72 hours incubation, the inner cell mass (ICM) and trophectoderm (TE) were differentially stained to determine the effect of AH on cell numbers.
Methods
1. Preparation of two-cell embryos
1) ICR mice
International Cancer Research (ICR) strain mice (females aged 4-5 weeks, males aged 10-15 weeks) with confirmed fertility were used in this study. Mice were exposed to a 12-hour light/dark cycle and room conditions were maintained at 22℃-25℃ and 40% to 60% humidity. There were no restrictions on diet or water supply.
2) Recovery of two-cell embryos and culture
For superovulation, intra-peritoneal injection of 7.5 IU pregnant mare's serum gonadotropin (PMSG, Sigma, St. Louis, MO, USA) and the intraperitoneal injection of human chorionic gonadotropin (hCG, Sigma) was performed 48 hours apart. Only female mice found to have a copulation plug 12 hours after mating were used in this study. Pregnant mice were sacrificed by cervical dislocation. Two-cell stage embryos were recovered from the oviduct, 46-50 hours after hCG injection, in a two-well dish (3260, Corning Costa, Union, CA, USA) under light microscopy: removed oviducts were suspended in Ham's F-10 (11150-043, Gibco, Grand Island, NY, USA) solution containing 10% serum substitute supplement (SSS, Irvine, Irvine, CA, USA) flushed with the same solution with a 30 gauge needle. The embryos were randomly divided into groups exposed to different AH procedures. The mean thickness of the ZP in each group is shown in Table 1.
On completion of each AH procedure, embryos were incubated for 24 hours in a 50 µL droplet of cleavage medium (CM, SAGE, Malov, Denmark), and then for a subsequent 48 hours in blastocyst medium (BM, SAGE). Blastocysts formed (Figure 1A, B) during embryo culture were double fluorescent stained to determine the cell numbers of the ICM and TE. Each culture medium was supplemented with 10% serum protein substitute (SPS, SAGE) and was used after 14 hours pre-incubation at 37℃ under 5% CO2 in air.

Comparison images of naturally-hatching and assisted hatching blastocysts, and differentially stained features of blastocyst in mouse (the scale bars are 50 µm, ×400). (A) Naturally hatching blastocyst (i.e., without assisted hatching). (B) Hatching blastocyst, with assisted hatching. (C) Differentially stained hatching blastocyst (inner cell mass, blue; trophectoderm, pink).
2. Mechanical Assisted Hatching (MAH)
1) Single MAH (sMAH)
In the center of a 60 mm Falcon dish (3002, Falcon, USA), each embryo was placed in a 20 µL drop of CM under mineral oil, and put into a 5% CO2 incubator at 37℃ to equilibrate. The laser system for ZP dissection was attached to an inverted microscope (TE2000-U, Nikon, Tokyo, Japan). On the left side of the micromanipulator (Nikon, Japan), the holding pipette (angle of 30°, Sunlight Medical, USA) was put in place. On the right side, the dissection pipette (partial zona dissection; PZD, angle of 30°, Sunlight Medical) was prepared, and the two pipettes were aligned parallel to each other.
Each two-cell stage embryo was placed in a drop of pre-equilibrated medium under oil and transferred to the microscopic stage. The holding pipette was placed in the 6 o'clock direction where the perivitelline space is widest. A PZD pipette penetrated the ZP from the 5 to 7 o'clock direction without damaging the blastomeres. ZP dissection was completed by rubbing the PZD pipette towards the 12 o'clock direction while maintaining the penetration after releasing the holding pipette (Figures 2A, 3).
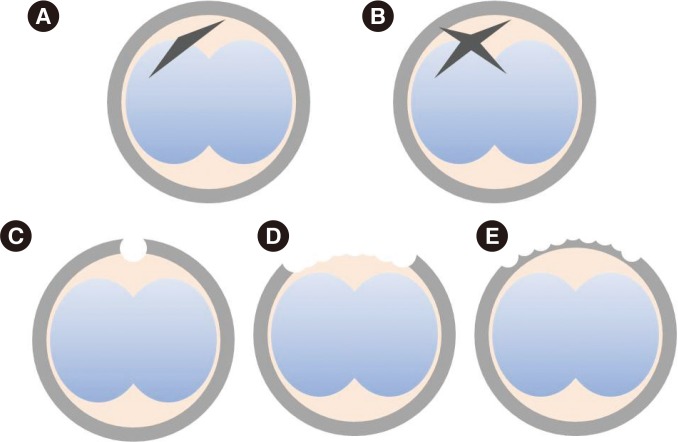
Schematic drawing of types of assisted hatching techniques. (A) Single mechanical assisted hatching. (B) Cross mechanical assisted hatching. (C) Single laser assisted hatching. (D) Quarter laser assisted hatching. (E) Quarter laser zona thinning-assisted hatching.
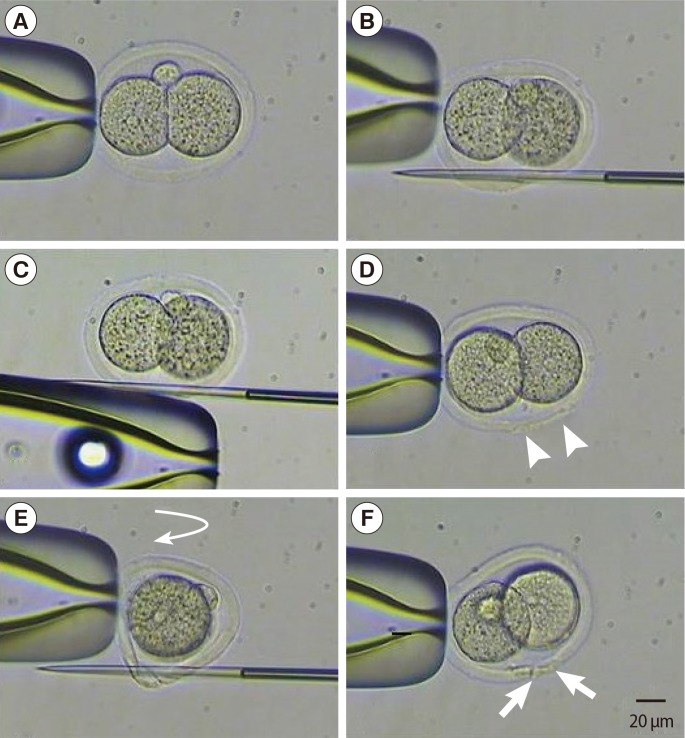
Photographs of techniques for sMAH and cMAH (the scale bars are 20 µm, ×400). (A) Mouse two-cell embryo fixed by holding pipette. (B) Entry position of the first cut for sMAH. (C) Embryos to rub holding pipette. (D) Mechanical assisted hatching. Arrow head, slit opening after sMAH. (E) Vertical rotation (curved arrow) of the embryo after the first slit is made and entry position of the second cut for the cross-shaped opening. (F) Mechanical assisted hatching. Arrow, slit opening after cMAH. sMAH, single mechanical assisted hatching; cMAH, cross mechanical assisted hatching.
2) Cross MAH (cMAH)
The same procedures described for sMAH were performed, and the dissection area on the ZP was placed facing the 6-o'clock direction. The holding pipette was then rotated 90 degrees, and another dissection was made to form a cruciate shape incision (Figures 2B, 3).
3. Laser assisted hatching (LAH)
An RI Saturn 5 active laser system attached to an inverted microscope (TE2000-U, Nikon) and an RI Viewer monitoring system with a display monitor (B19940, Samsung, Suwon, Korea) was used for LAH. A non-contact-type laser at a wavelength of 1.48 µm was emitted from the infrared diode through the object glass. A red pilot spot on the display monitor indicated the area where the hole in the ZP would be produced, and the laser size and location were adjusted by a computer mouse connected to the system.
1) Single LAH (sLAH)
Five to six two-cell embryos were placed in a drop of CM under oil, and were placed on the stage of an inverted microscope. A laser beam was emitted on the area where the ZP was furthest away from the blastomeres (Figure 2C). The emitted light beam was 9-13 µm in diameter, and the drilled hole was 0.5-1 µm larger than the thickness of the ZP.
2) Quarter LAH (qLAH)
Through the display monitor, a virtual curved line was drawn following the outer contour of the ZP (Figure 2D), and a continuous laser beam was emitted tracing the line, to drill a hole in about a quarter of the ZP surface. The laser was emitted at the point where the blastomeres were far apart. The size of the laser was identical to that used in the sLAH procedure mentioned above, and 8-15 emissions were required to complete the qLAH procedure.
3) Quarter laser zona thinning assisted hatching (qLZT-AH)
Similar to the method described for qLAH, a virtual curved line following the outer contour of the ZP was made, and the thickness of ZP was reduced by approximately 50%-80% in a quarter of the ZP surface. The size of the laser emitted was about 5-10 µm in diameter, and 8-15 emissions were required to complete the procedure (Figure 2E).
4. Double immunofluorescence staining of blastocyst
Double immunofluorescence staining of the embryo was performed using the protocol described by Park et al. [22]. In brief, following a 72 hours incubation period, solution 1 (a mixture of Ham's F-10, 1% Triton X-100 [T9254, Sigma], and 100 µg/mL propidium iodide [P4170, Sigma]) and solution 2 (a mixture of 99.9% ethanol [Merck, Germany], 25 µg bisbenzimide [B2883, Sigma]) were used for staining. The blastocyst embryo was stained following exposure to solution 1 for 15 second, and to solution 2 in 4℃ atmosphere for 2 hours. The stained blastocyst was washed in glycerol (G2025, Sigma) and transferred to a slide glass for inspection under a fluorescent microscope (BX50, Olympus, Tokyo, Japan) (Figure 1C).
5. Statistical analysis
The BFR is reported in percentage values. Comparison of standard deviations of discrete variables between groups was performed with a chi-squared test using Microsoft Excel 2007. Differences in the blastocyst cell number between two selected groups were analyzed by the Student's t-test. A value of p<0.05 was defined to be statistically significant.
Results
1. The effect of various two-cell embryo AH methods on mouse embryo development
Mouse two-cell embryos were retrieved after 5 AH methods that used either micropipettes (sMAH, cMAH) or a laser (sLAH, qLAH, qLZT-AH) and were followed by a 72 hours incubation. Rates of blastocyst development are shown in Table 2. After 48 hours incubation, the hatching BFR was highest in the sLAH group. After 72 hours incubation, the hatched BFR was highest in both the qLAH and qLZT-AH groups. There were no hatched blastocysts in the 67 two-cell embryos that underwent sMAH, and the hatched BFR was significantly lower than that of the control group.
2. The effect of various two-cell embryo AH methods on blastocyst cell number
Double immunofluorescence staining was performed on the 72-hour incubated embryos to obtain differential cell number analysis of the ICM and TE. The results are shown in Table 3. Initially, 330 blastocyst embryos were prepared for staining; however, 10 embryos were lost, and embryos that were either partially stained, stained with a single agent, or failed to clearly distinguish ICM and TE were regarded as staining failure and excluded from analysis. The remaining 276 embryos are included in Table 3. Both the cell number in the ICMs and the proportion of ICM in the embryo were higher in the sMAH and control group compared to the other group. The cell number in the TE was highest in the cMAH and qLAH groups. The qLAH showed the highest total cell number.
Discussion
AH is a very aggressive method compared to other ART, and its clinical efficacy remains controversial. Choosing the ideal AH method varies between researchers, although the hatching process is essential prior to implantation. Schiewe et al. [23] reported that without the addition of serum, the basic culture media (mHTF; modified Human Tubal Fluid, Irvine) suppressed natural hatching, while after AH, the blastocyst formation rate was significantly higher despite the absence of serum (88% and 2%, with and without AH, respectively). Makrakis et al. [16] noted that mechanical AH is superior to chemical AH because the latter uses acidic solution and carries a potential risk of embryo damage. Cieslak et al. [17] proposed a 3-dimensional, cross-shaped dissection of the ZP using mircopipettes, by dissecting the ZP twice after embryo rotation. A higher pregnancy rate was reported following this method compared to conventional AH (42.0% vs. 33.3%). In the present study, the effect of methods similar to those described by Cieslak et al. [17] on the embryos was investigated. The hatching BFR after 72 hours incubation was 77.6% in the sMAH. The hatched BFR was significantly higher in the cMAH group compared to the sMAH group (0% vs. 9.0%). The cross-shaped mechanical AH procedure requires a higher proficiency from the operator, and is required to be completed within 1 minute. In the present study, all sMAH and cMAH procedures were accomplished within 1 minute and there were no damaged blastomeres or dead embryos.
LAH was suggested by Strohmer and Feichtinger [19] in order to overcome the problems encountered with the pre-existing AH methods. A laser beam was emitted 1-3 times to drill the ZP. Hsieh et al. [24] also reported that LAH resulted in higher rates of pregnancy (31.8% vs. 16.12%) and implantation per embryo (8.2% vs. 3.8%) compared to chemical AH. In human IVF, Ghobara et al. [20] compared 1.5 and 5 times of 8 µm laser emissions in LAH. Both implantation and pregnancy rate were significantly higher in the widely-excised group. Khalifa et al. [14] also reported that wide-diameter excision of the ZP was beneficial in blastocyst embryo hatching. The results of our study are similar to previous studies, in that the groups with the highest hatched BFRs (49.1% and 40.4% in the, qLAH and qLZT-AH groups, respectively) were the groups in which the ZP was extensively excised.
The laser used in our study was not fixed in a single position; rather, the area of emission displayed on the monitor attached to the computer was able to be adjusted. The laser beam could be aimed at large areas (either linear or curved), and the LAH procedure could be finished in a time as short as approximately 10 seconds. LAH is beneficial, since while it maintains an effect equivalent to that of using acidic Tyrode's solution, the risk of damaging the embryo with acidic solution is eliminated. In patients who have undergone unsuccessful IVF more than twice, Petersen et al. [21] reported a significantly higher implantation rate in the patients whose embryos underwent LAH than in those whose embryos did not (10.9% vs. 2.5%). In patients whose embryos underwent LAH, the pregnancy rate was significantly higher with thin-ZP embryos (thinner than 16 µm) compared to thick-ZP embryos (thicker than 17 µm) (69% vs. 25%). ZP thickness could therefore serve as a criterion for patient selection for LAH [25].
Clinically, most AH is performed on embryos after three-day culture, when the embryo is in the cleavage (4-16 cell stage). This is because embryo grading must precede embryo selection (selective AH). The present study also initially aimed to use the cleavage-stage mouse embryos. However, the rate of embryo development varies highly after two-cell stage, and the embryos tend to proceed to the morula stage immediately after the eight-cell stage, making it difficult to harvest sufficient number of cleavage-stage embryos for investigation. Thus, only two-cell embryos were selected, in order to standardize the embryo conditions. In addition, in order to avoid unexplained two-cell block in the mouse, two-cell embryos were retrieved 48 hours after natural mating.
In a study of bovine blastocysts, Iwasaki et al. [26] concluded, using double immunofluorescent staining, that the total cell count in the blastocyst (75±27) was higher in embryos cultured inside the rabbit oviduct compared to those cultured in vitro culture (44±18). The data from the control group in our study are similar to those in a study of two-cell mouse embryos by Thouas et al. [27] (ICM, 21±2; TE, 55±4; total, 75.3±3). Park et al. [28] failed to show a differential effect of glucose concentration in the CM (0.5 M vs. 3.15 M) on hatched blastocyst formation, total cell count, or ICM cell count. Park et al. [29] found significant differences in the cell number in the ICM and TE, but no difference in total cell count following the addition of energy sources to the media at different stages of embryonic development, and concluded that the total cell count is not affected by embryo development rate (EDR).
The hatching of the ZP is followed by secretion of various proteases from the TE, weakening the ZP [23,30]. In the present study, the hatched BFR (after 72 hours incubation) was significantly higher in the two groups in which a larger proportion of the surface (one quarter) of the ZP was either dissected or thinned. The higher cell number of the TE did not appear to augment the hatching process, since despite the higher hatched BFR in the qLZT-AH group than in the cMAH group, the TE cell number was higher in cMAH group than in the qLZT-AH group.
Iwasaki et al. [26] showed that rapid embryo development results in a higher cell count and a higher ICM ratio (The ratio of the cell count in the ICM to the total cell number). In our study, in the qLAH group, in which the hatched BFR was highest, the EDR was also highest, but in contrast the ICM ratio was lower. Further study is needed to determine whether this is due to the AH.
In contrast, there have been reports that blastocyst grade is not altered by cell number, but by the shape of the embryo. In a study of human IVF, in which only blastocysts were implanted, Richter et al. [31] showed no difference in implantation rate according to the size of the blastocyst, TE cell number, or the size and shape of the ICM. They concluded that the cell count has little value in identifying the embryos that might result in higher implantation rates. Lane and Gardner [32] also showed that the ICM cell count was not significantly different between implanted and implantation failure groups. In the mouse, the TE cell number did not positively correlate with the implantation rate, or blastocyst cell count [32].
In the current study, results of a 72 hours culture following the sMAH and cMAH methods (using micropipette) and the sLAH, qLAH, qLZT-AH methods showed that the sMAH method resulted in a lower hatched BFR, and that while cMAH and sLAH did not show a significant difference in hatched BFR to controls, qLAH and qLZT-AH resulted in a higher hatched BFR. The number of TE cells and the total number of cells were higher in the qLAH group than in other groups. The qLAH method was found to be effective for development in mouse two-cell embryos, and further study on human IVF material is now required.
Notes
No potential conflict of interest relevant to this article was reported.