Relationship between hematologic parameters related to systemic inflammation and insulin resistance-associated metabolic parameters in women with polycystic ovary syndrome
Article information
Abstract
Objective
The aim of the present study was to evaluate the associations between hematologic parameters related to systemic inflammation and insulin resistance-associated metabolic parameters in women with polycystic ovary syndrome (PCOS).
Methods
Eighty-two women between the ages of 18 and 35 years who were diagnosed with PCOS were included in this study. A 2-hour 75-g oral glucose tolerance test (OGTT) was administered to all study participants; fasting and postprandial glucose and insulin levels were measured simultaneously during the 2-hour OGTT. Hematologic parameters were derived from a standard complete blood count and a differential count of fasting-state blood samples. The correlations between hematologic parameters and insulin resistance-associated clinical and metabolic parameters were evaluated using the Spearman rank correlation and partial correlation coefficients. Hematologic parameters related to systemic inflammation were compared between the two groups, categorized by the presence or absence of insulin resistance.
Results
Significant differences in the absolute neutrophil count, absolute monocyte count, platelet count, and neutrophil-lymphocyte ratio were found between the insulin-resistant group and insulin-nonresistant group. Correlation analysis found that all hematological parameters, except for the platelet-lymphocyte ratio, were associated with at least one insulin resistance-associated metabolic parameter. However, these significant correlations between hematological and metabolic parameters were attenuated after controlling for the effects of other covariates using partial correlation analysis.
Conclusion
The association between hematologic parameters indicative of systemic inflammation and insulin resistance-associated metabolic parameters seems to be strongly influenced by other anthropometric covariates in women with PCOS.
Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrine disease and affects between 5% and 10% of reproductive-age women [1]. PCOS is a complex disease associated with both reproductive problems and metabolic disturbances. Insulin resistance is the cardinal component involved in the pathogenesis of metabolic disorders such as impaired glucose tolerance (IGT), type 2 diabetes mellitus (T2DM), dyslipidemia, and cardiovascular disease in PCOS [2,3]. Although the pathophysiology of insulin resistance has not been clearly established, it is known that systemic inflammation is an important factor in triggering insulin resistance [4-6].
Systemic inflammation is associated with the pathogenesis of a variety of chronic diseases. C-reactive protein (CRP) is a very useful and commonly used parameter for evaluating systemic inflammation [7,8]. Hematologic parameters derived from the complete blood count and differential count have recently emerged as indicators of systemic inflammation due to their cost-effectiveness and convenience [9,10].
Several studies have confirmed the relationship between hematologic parameters associated with systemic inflammation and metabolic parameters indicating insulin resistance [11-13]. However, most studies have been conducted among patients who have metabolic diseases, such as coronary heart disease or T2DM. To our best knowledge, studies on insulin resistance and inflammation in women with PCOS are lacking or inconclusive [10,14-16].
Therefore, the purpose of this study was to evaluate the association between hematologic parameters indicative of systemic inflammation and insulin resistance-associated metabolic parameters in women with PCOS.
Methods
1. Participants
Korean women between the ages of 18 and 35 years who were newly diagnosed with PCOS at Inje University Haeundae Paik Hospital from January 2010 to December 2013 were recruited for this study. Among patients previously diagnosed with PCOS according to the 2003 Rotterdam criteria, those who met the diagnostic criteria of the recently revised international consensus guidelines for PCOS were included in this study, after the exclusion of patients with other etiologies (congenital adrenal hyperplasias, androgen-secreting tumors, and Cushing's syndrome) [17,18]. Irregular menstrual cycles were defined as menstrual cycles longer than 35 days or fewer than 21 days, or longer than 90 days for any one cycle, or less than eight cycles per year in women with PCOS [18]. Primary amenorrhea by age 15 or >3 years post-thelarche was also included in the category of irregular menstrual cycles. Clinical hyperandrogenism was defined by the presence of hirsutism (modified Ferriman-Gallwey score >6) [18], and biochemical hyperandrogenism was defined as an elevated serum androgen level beyond the 95% confidence limits defined in controls in a study conducted on Korean women with PCOS (total testosterone >0.68 ng/mL and/or free testosterone >1.72 pg/mL) [19]. Patients who were previously or newly diagnosed with diabetes, thyroid disease, or hyperprolactinemia; those with a history of ovarian surgery, use of a medication known to affect sex hormone or gonadotropin levels within 6 months of enrollment in the study (e.g., oral contraceptives, ovulation induction agents, glucocorticoids, or antiandrogens); and those who were taking antidiabetic drugs, including insulin sensitizers, were excluded from this study [20-23]. This retrospective study was approved by the Institutional Review Board of Inje University Haeundae Paik Hospital (IRB No. 129792-2014-035), which waived the requirement for patient informed consent in the present study.
2. Measurement of anthropometric parameters and ultrasound examinations
Clinical variables such as age, parity, height, body weight, body mass index (BMI), waist circumference, hip circumference, and waist-to-hip ratio (WHR) were evaluated for all study participants when they first visited the outpatient department. Pelvic ultrasound examinations (transvaginal or transrectal [24]) were conducted in the early follicular phase using a Voluson LOGIQ S7 (GE Ultrasound Korea Ltd.) equipped with a microconvex intracavitary probe with a frequency range of 3.6 to 9.0 MHz. Transvaginal ultrasound was performed in all patients except for eight patients who underwent transrectal ultrasound because they were virgins without coital history. Polycystic ovarian morphology was defined as the presence of over 20 follicles (2 to 9 mm in size) and/or an ovarian volume of over 10 mL [17,18,24]. All ultrasound examinations were performed by the same expert in ultrasound for reproductive endocrinology based on the international consensus on ultrasound assessment of PCOS [25].
3. Biochemical measurements and determination of hyperglycemia
This study was conducted according to the guidelines of the Declaration of Helsinki. Blood samples for laboratory analyses were taken from all subjects in the early follicular phase after overnight fasting. Hematologic parameters, including the white blood cell count, absolute neutrophil count (ANC), absolute lymphocyte count, absolute monocyte count (AMC), and platelet count (PC), were derived from a standard complete blood count and a differential count of fasting-state blood samples. The hematologic parameters were calculated from a combination of these parameters, such as the neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR), and lymphocyte-monocyte ratio. Serum glucose and insulin levels were analyzed using an L-Type GluI device (Wako) and an Elecsys Insulin assay (Roche), respectively. Cholesterol and triglyceride levels were measured using Pureauto S (Sekisui), and serum high-density lipoprotein and low-density lipoprotein levels were measured using Cholestest (Sekisui). Both intra- and inter-assay coefficients of variation for all assays were below 8%. Postprandial glucose and insulin levels were measured 60 and 120 minutes after glucose ingestion during a 2-hour 75-g oral glucose tolerance test.
In the present study, hyperglycemia, including prediabetes (high fasting glucose or IGT) and diabetes, was diagnosed based on the American Diabetes Association criteria of fasting glucose ≥100 mg/dL or 2-hour postload glucose ≥140 mg/dL [23,26].
4. Assessment of insulin sensitivity and determination of insulin resistance
Insulin sensitivity assessment indices (ISAIs) were calculated for all study participants. Established fasting ISAIs derived from a combination of fasting insulin and glucose levels were calculated as follows [20-23]: homeostatic model assessment of insulin resistance (HOMA-IR) was calculated as the glucose level (mg/dL)×insulin level (μU/mL)/405; the glucose-to-insulin ratio (GIR) was calculated by dividing the glucose level (mg/dL) by the insulin level (μU/mL); and quantitative insulin sensitivity check index (QUICKI) was calculated as 1/{log[insulin level (μU/mL)]+log[glucose level (mg/dL)]}. Patients with PCOS who showed abnormal levels for at least one of the established ISAI criteria in previous studies were defined as having abnormal insulin sensitivity: fasting insulin ≥15 µIU/mL [27], HOMA-IR ≥2.64 [28], GIR ≤10.7, or QUICKI ≤0.34 [29]; and postprandial 2-hour insulin ≥45 µIU/mL [30]. In the present study, insulin resistance was determined as the presence of abnormal insulin sensitivity or hyperglycemia [23].
5. Statistical analyses
Values are expressed as the mean±standard deviation. The correlations between hematologic parameters and insulin resistance-associated clinical and metabolic parameters were evaluated using Spearman rank correlation coefficients and linear regression analysis, with partial correlations used to control for the effects of other confounding covariates such as age, BMI, and WHR. The Mann-Whitney U test was used to compare hematologic parameters between the two groups categorized according to the presence or absence of insulin resistance. All statistical analyses were conducted using SPSS version 25.0 (IBM Corp.), with p<0.05 considered to indicate statistical significance.
Results
In total, 82 patients with PCOS were enrolled in the present study. Table 1 shows the baseline clinical and biochemical characteristics of the study participants. Table 2 shows a comparison of hematologic parameters between those with insulin resistance and those without insulin resistance among patients with PCOS. ANC, AMC, PC, and NLR were significantly higher in the insulin-resistant group than in the insulin-nonresistant group.
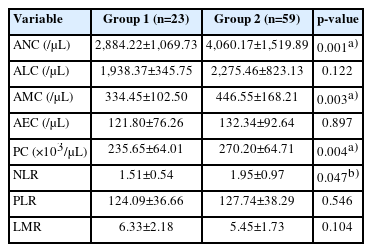
Comparisons of hematologic parameters derived from the white blood cell count and differential count between those with insulin resistance and those without insulin resistance among patients with polycystic ovary syndrome
In the correlation analysis, all hematological parameters except for the PLR were associated with at least one insulin resistance-associated biochemical metabolic parameter (Table 3). In particular, ANC showed significant positive correlations with fasting and postprandial glucose and insulin levels. Additionally, ANC showed significant correlations with all fasting-state ISAIs, including a positive correlation with HOMA-IR and negative correlations with GIR and QUICKI. However, these significant correlations between hematological and metabolic parameters were attenuated after controlling for the effects of other covariates, including age, BMI, and WHR, using partial correlation analysis (Table 4).
Discussion
PCOS is not only an endocrine disorder but also a metabolic disorder that consequently contributes to lifetime health risks. Inflammation and insulin resistance are cardinal components in the pathogenesis of PCOS [30]. The association between these two factors has been proven through several studies in patients with metabolic diseases such as metabolic syndrome and T2DM [4,11-13]. To date, however, few studies have been conducted on the relationship between these two factors in patients with PCOS [16]. Therefore, we aimed to evaluate the relationship between hematologic parameters related to inflammation and insulin resistance in patients with PCOS. In the present study, ANC, AMC, PC, and NLR were significantly higher in the insulin-resistant group than in the insulin-nonresistant group, and most systemic inflammation-related hematologic parameters were significantly associated with at least one insulin resistance-associated metabolic parameter in women with PCOS. However, most of these strong associations were substantially attenuated after controlling for covariates such as age, BMI, and WHR.
As previously mentioned, the association between insulin resistance and inflammation has been demonstrated in several studies in patients with metabolic diseases such as T2DM and glucose intolerance [4,11-13,31,33]. Lou et al. [12] found a significant positive correlation between the NLR value and insulin resistance in patients who were newly diagnosed with T2DM, and they suggested that the NLR value can be a predictive and prognostic marker for insulin resistance.
To date, few studies have investigated the relationship between insulin resistance and chronic systemic inflammation in women with PCOS, and the results of those studies have been inconsistent [9,10,11,14-16]. Ozay and Ozay [10] compared metabolic and hormonal factors and inflammatory markers between 110 PCOS patients and 135 healthy women. They noted that the neutrophil count and PC were significantly higher in patients with PCOS, and these two parameters were also significantly correlated with BMI and WHR, similar to the findings of our study. In the study of Yilmaz et al. [34], the NLR and neutrophil count were found to be significantly higher in patients with PCOS. In a subgroup analysis, the obese PCOS group had higher insulin and HOMA-IR levels than the controls, and the NLR was positively correlated with HOMA-IR, high-sensitive CRP, BMI, waist circumference, and insulin levels [34].
Although the association between inflammatory markers and insulin resistance has been demonstrated in PCOS patients, whether obesity is an independent factor in this association remains a matter of debate. In our study, the significant correlation between systemic inflammation-related markers and insulin resistance-associated metabolic parameters in women with PCOS was strongly attenuated after controlling for the effects of age and other anthropometric parameters. Pergialiotis et al. [16] conducted a study of inflammatory markers in 266 PCOS patients. Consistent with our study, significant positive correlations were found between metabolic parameters and hematologic parameters such as the NLR and PLR. However, contradictory to our results, these associations did not change after adjusting for confounding effects due to BMI. Cakiroglu et al. [9] also suggested that the NLR and PLR were significantly elevated in all PCOS subjects, but this increase was independent of the effect of obesity, which is not in agreement with our findings. The discrepancy in the results between these two studies and ours remains difficult to explain, and whether the impact of a chronic systemic inflammatory state on the phenotypic features of PCOS is due to its own pathophysiology or due to factors such as comorbid obesity or age remains unclear. One possible explanation is that serum hormones such as androgens have an additional effect on the confounding effect of age and body weight. Blood androgen levels have been noted to be significantly correlated with inflammatory markers in PCOS patients. For example, a study suggested that androgens trigger inflammatory cells and initiate the inflammatory process [14]. Zeng et al. [35] suggested that hyperandrogenism, insulin resistance, and obesity form a vicious cycle to promote PCOS development. In women with PCOS, the prevalence of hyperandrogenism differed according to the ethnicity of the study participants [19], and it is possible that these differences in hormonal patterns of study participants resulted in the discrepancy in the confounding effects of age and BMI between other studies and ours.
De Luca and Olefsky [4] reported the mechanism by which systemic inflammation leads to insulin resistance and suggested that obesity is an important factor involved in inflammation, which strongly supports our results.
CRP is a very useful and commonly used parameter for evaluating systemic inflammation. In studies of PCOS patients, CRP has also been widely used as a parameter reflecting systemic inflammation. However, a CRP test is comparatively expensive, so it is not routinely measured in the general population. In the present study, we used systemic inflammatory markers derived from the complete blood count, which is less expensive and much more commonly assessed than CRP.
Our study has several limitations, including a retrospective study design and a relatively small sample size. When the sample size was calculated with reference to a previous study [11], the sample size was calculated as 41 people in each group (82 people in total) with an effect size=0.629, a significance level α=0.05 and a power of (1-β)=0.80.
As mentioned in the ‘discussion,’ this study did not separately evaluate hormonal effects on the confounding effects of covariates such as age, BMI, and WHR, which could be another drawback of the study
In conclusion, the association between hematologic parameters indicating chronic systemic inflammation and insulin resistance-associated metabolic parameters seems to be strongly influenced by other anthropometric covariates in women with PCOS. Further prospective large-scale trials on the relationship between insulin resistance and inflammation with additional analyses including hormonal factors are needed to clarify these preliminary findings.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Author contributions
Conceptualization: MC, SK, SC. Data curation: SC. Formal analysis: SC. Methodology: MC, SC. Project administration: SC. Visualization: MC, SC. Writing-original draft: MC. Writing-review & editing: MC, SK, SC.



