Effects of controlled ovarian stimulation regimens on top-quality blastocyst development and perinatal outcomes with the freeze-all strategy: A retrospective comparative study
Article information
Abstract
Objective
This study aimed to determine the effect of ovarian stimulation regimens on the top-quality blastocyst development rate and perinatal outcomes with the freeze-all strategy.
Methods
A retrospective comparative cohort analysis of 149 in vitro fertilization (IVF) cycles using the freeze-all strategy was conducted. The IVF cycles were stimulated with either a gonadotropin-releasing hormone antagonist or clomiphene citrate along with gonadotropin based on the patient’s serum anti-Müllerian hormone level. Oocyte retrieval, fertilization, and embryo culture were performed following standard procedures. All good-quality blastocysts were cryopreserved and used for frozen-thawed embryo transfer (FET) in subsequent cycles. The fertilization, blastulation, and top-quality blastocyst development rates were calculated. The perinatal outcomes of FET cycles, gestational period, and birth weight were assessed.
Results
The main outcome of this study was the top-quality blastocyst development rate, and the secondary outcomes were perinatal parameters (e.g., gestational period and birth weight) between the stimulation regimens. Despite the higher number of usable-quality embryos in the antagonist group, the blastocyst development rate remained comparable (p=0.105). Similarly, perinatal outcomes were comparable in subsequent FET cycles (p=0.538).
Conclusion
These findings suggest that the choice between antagonist and clomiphene citrate with gonadotropin as stimulation in controlled ovarian stimulation regimens may not affect the top-quality blastocyst development rate. The IVF outcomes (e.g., clinical pregnancy, miscarriage, and live birth rates) remained unaffected in subsequent FET cycles. Unlike fresh embryo transfer, the birth weight and gestational length were not associated with prior controlled ovarian stimulation regimens when the freeze-all strategy was used.
Introduction
Assisted reproductive technologies are widely used for the treatment of infertility/subfertility. The first live birth using in vitro fertilization (IVF) was achieved through natural-cycle IVF. Later, to increase the number of recruited follicles per cycle and to prevent the spontaneous surge of luteinizing hormone (LH), gonadotropin-releasing hormone (GnRH) analogs were introduced into controlled ovarian stimulation (COS). The use of these agents in COS led to increases in the number of oocytes retrieved and fertilized embryos per cycle. The availability of multiple embryos provides the ability to select the best-quality embryo for transfer, leading to an increased success rate. Moreover, surplus good-quality embryos can be cryopreserved and used for future transfer [1]. COS is aimed at the recruitment of multiple follicles and the inhibition of spontaneous ovulation simultaneously. To increase the oocyte yield per cycle, gonadotropins have been used [2], and GnRH analogs (agonists and antagonists) are used to prevent the LH surge [3]. The estimated pregnancy rate per started cycle with COS is approximately 20% to 30% [4]; however, when using minimal ovarian stimulation, only about a 10% pregnancy rate per stimulated cycle is observed [5]. This difference in pregnancy rates highlights the importance of COS for the success of assisted reproductive technologies. To retrieve multiple mature oocytes in a single cycle, high doses of exogenous gonadotropins are administered that stimulate the development of multiple oocytes, leading to their maturity. Ovarian stimulation is a major component of the IVF procedure; however, aggressive ovarian stimulation using higher doses has negative effects on oogenesis, embryo quality, endometrium receptivity, and possibly the perinatal outcomes of IVF [6]. Therefore, it is necessary to develop an ideal IVF protocol that aims at providing good-quality multiple embryos, a high chance of good-quality embryo transfer with a low cycle cancellation rate, a high pregnancy success rate, fewer side effects, lower costs, and fewer required hospital visits.
Clomiphene citrate (CC) is an ovarian stimulation agent approved by the U.S. Food and Drug Administration in 1961 that is commonly used in minimal stimulation in patients with a poor ovarian response along with gonadotropins and GnRH antagonists (GnRHa) [7]. This drug exerts an antiestrogen effect on the pituitary gland, primarily by binding to the estrogen receptors in the hypothalamus, thereby releasing follicle-stimulating hormone (FSH) to produce more follicles and complementing the activity of externally administered gonadotropins. Simultaneously, it inhibits the release of LH, thereby preventing a premature LH surge, which is responsible for premature ovulation [8]. CC is orally administered, inexpensive, and easily available. Co-administration of CC and human menopausal gonadotropin (hMG) (CC+hMG) reduces the gonadotropin dose requirement, particularly for patients who prefer fewer injections. The extended use of CC until the day of ovulation triggering has been advised recently, as CC exerts antiestrogenic activity to prevent a spontaneous LH surge, enabling it to replace GnRHa or GnRH agonists at a lower cost [8,9]. However, due to its antiestrogen effects on reproductive organs, its prolonged use poses serious concerns, since it may affect endometrial receptivity in fresh embryo transfer cycles. Some additional disadvantages are associated with this stimulation protocol, such as a lower number of oocytes retrieved and, sometimes, cycle cancellation.
GnRHa has recently been applied in clinical practice for COS in IVF. GnRHa-based stimulation protocols offer several advantages over GnRH agonist use. These advantages include a shorter duration of treatment, a shorter duration of FSH administration, and a lower risk of ovarian hypersensitivity syndrome (OHSS) [10]. In addition, the GnRHa protocol overcomes some of the disadvantages associated with the GnRH agonist protocol, such as lower oocyte yield and serum estradiol (E2) levels on the ovulation trigger day [11]. However, the results of the previous study have highlighted the effectiveness of the GnRHa protocol in COS in terms of higher fertilization rates, mean numbers of transferrable-quality embryos, and successful pregnancy rates, as well as a lower incidence of OHSS [11].
Previous studies have suggested that ovarian stimulation protocols are associated with top-quality blastocyst development and an increased risk of adverse perinatal outcomes, such as lower birth weight and preterm delivery, in fresh embryo transfer cycles [12]. However, it remains unknown whether the top-quality blastocyst development after COS differs between the distinct stimulation regimens. Whether an increased risk of adverse perinatal outcomes still exists even after using frozen-thawed embryo transfer (FET) in subsequent cycles is an intriguing question. The present study was undertaken to evaluate the effects of COS with CC+hMG and GnRHa regimens on oocyte and embryo quality and subsequent FET outcomes. The main outcome of this study was the top-quality blastocyst development rate between the COS regimens. The secondary outcomes were fertilization rate, clinical pregnancy, miscarriage, and live birth rates between these two groups.
Methods
1. Patient selection
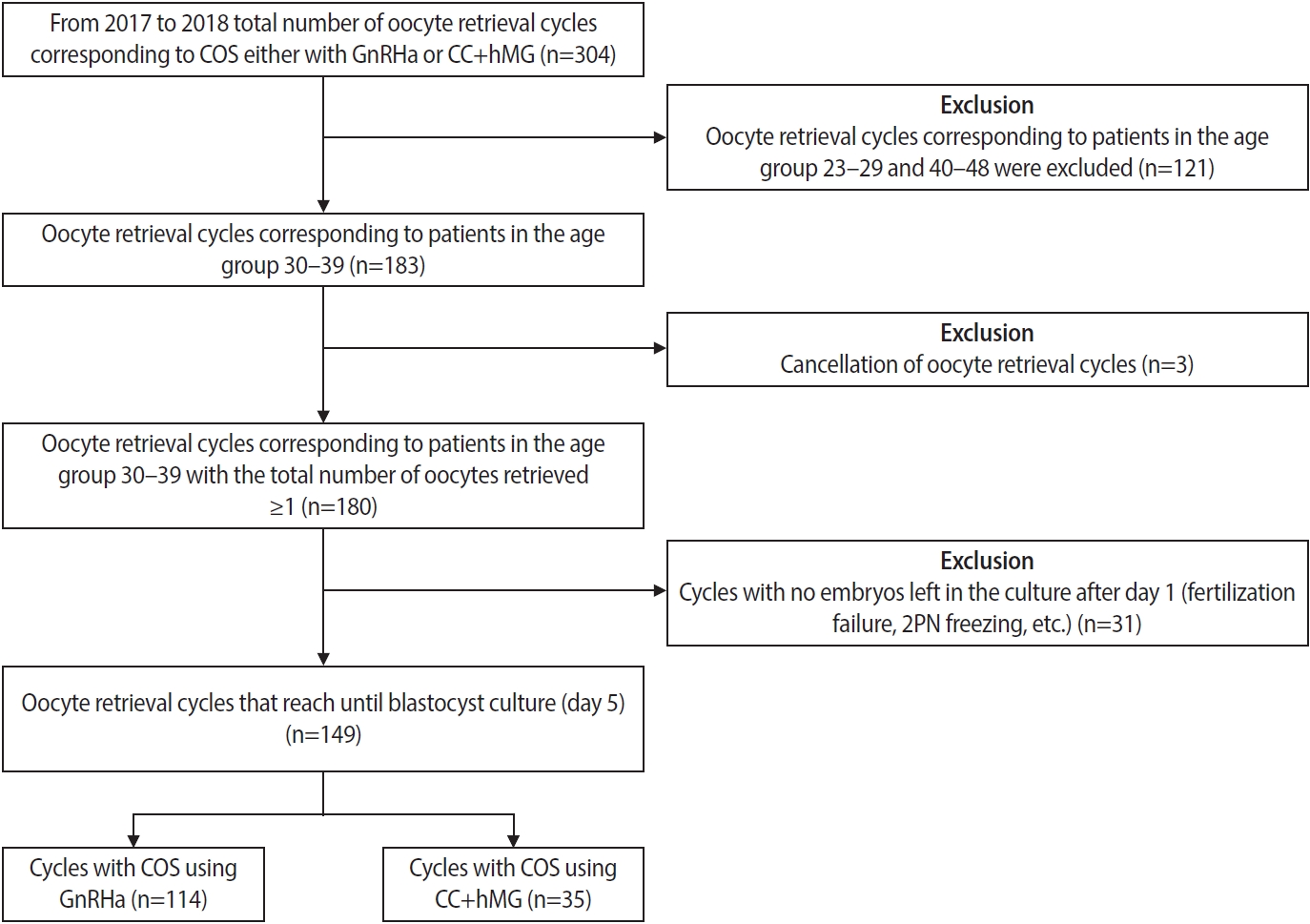
This is a retrospective cohort analysis of a total of 118 patients (149 IVF cycles) 30 to 39 years of age with a diagnosis of infertility treated at Kinoshita Ladies Clinic between June 2017 and December 2018. The inclusion criteria were an indication of IVF or intracytoplasmic sperm injection (ICSI) and COS with a GnRHa or CC+hMG protocol (Figure 1). All the patients provided written informed consent for their’ anonymized medical records to be used for clinical research purposes, and the study was approved by the Institutional Ethics Committee of Kinoshita Ladies Clinic (approval number: 006).
2. COS protocols
At the start of the cycle, serum anti-Müllerian hormone (AMH) levels were measured. Patients with serum AMH levels >1.0 ng/mL were stimulated with GnRHa, whereas patients with serum AMH levels ≤1.0 ng/mL were stimulated with a mild stimulation protocol using CC+hMG.
1) GnRHa protocol
Dose adjustments during treatments were chosen on a case-by-case basis according to patients’ characteristics. A dose of 0.25 mg of GnRHa (Cetrotide, Merck BioPharma Co. Ltd.) every other day and 150 to 450 IU/day of hMG (HMG Ferring, Ferring Pharmaceuticals Co. Ltd.) was started on day 3 to 5 of the menstrual cycle. When a leading follicle reached a diameter of ≥18 mm, ovulation was triggered with a GnRH agonist nasal spray (Buserecur 1.25 mg, Fuji Pharma Co. Ltd.). In patients at high risk for OHSS, ovulation was triggered with 1.25 mg GnRH agonist and 5,000 IU (intramuscular) of human chorionic gonadotropin (hCG) (Ovidrel, Merck Biopharma Co. Ltd.). Transvaginal ultrasound (TVS)-guided oocyte retrieval was performed 34 to 38 hours after the trigger, following the standard procedure.
2) CC+hMG protocol
For COS with the CC+hMG protocol, on day 3 of the menstrual cycle, serum basal FSH, LH, E2, and progesterone (P4) levels were assayed. From menstrual cycle days 3 to 5 onwards, patients in this group were given 50 to 100 mg/day of CC (Clomid, Fuji Pharma Co. Ltd.) and 150 IU/day of hMG for about 10 days. Cycle monitoring was started on days 7 to 8 of the menstrual cycle, and then TVS was performed every 2 to 4 days to adjust the hMG dose according to follicle development. During each monitoring, the number(s) and sizes (mm) of follicles were recorded by TVS, along with measurements of serum FSH, LH, E2, and P4 levels on the same days. The final stage of oocyte maturation was induced by an intramuscular injection of a 10,000-IU hCG trigger (HCG Mochida, Mochida Pharmaceutical Co. Ltd.), once at least one follicle reached 18 mm or greater in diameter. TVS-guided oocyte retrieval was performed 34 to 38 hours after the trigger. Attempts were made to retrieve all follicles more than 10 mm in diameter.
3. Oocyte fertilization, embryo culture, and transfer
Based on semen parameters, the previous history of failed IVF, the total number of oocytes retrieved, and other relevant factors, either ICSI or a split protocol (conventional-IVF+ICSI) was used for fertilization. For ICSI, oocytes were denuded using hyaluronidase (80 IU/mL) (Fujifilm, Irvine Scientific Inc.) to inspect the extrusion of the first polar body, and metaphase II (MII) oocytes were subjected to sperm injection, as described elsewhere [13]. The fertilization of oocytes was determined by the presence of two pronuclei and polar bodies 18 to 20 hours after insemination. After fertilization, zygotes were cultured in 25 µL of one-step medium (Naka Medical Corp.) under mineral oil (Fuso Pharmaceutical Industries Ltd.) at 37 ºC and 6% CO2, 5% O2, and 89% N2. Additionally, an examination for the number and regularity of blastomere and embryonic fragmentation was performed on day 3 embryos, and scoring of cleavage stage embryos was performed according to Veeck’s classification [14]. On the morning of days 5 and 6, the development of blastocysts was reviewed and recorded using the Gardner criteria by trained embryologists at the Kinoshita Ladies Clinic [15]. Day 5 and 6 blastocysts were rated based on: (1) the degree of expansion and hatching status (1=early blastocyst, less than half volume of the embryo is occupied by the blastocoel; 2=blastocyst, more than half of the volume is occupied by the blastocoel; 3=full blastocyst, the entire volume of the embryo is occupied by the blastocoel, 4=fully expanded blastocyst, the blastocoel volume is larger than the previous stage embryo and thinning of the zona pellucida has started; 5=hatching blastocyst, herniation of the trophectoderm [TE] has started through the zona layer; 6=hatched blastocyst, the blastocyst has completely escaped from the zona). For blastocysts graded as 3 to 6 (that is, from full blastocyst onwards), the further assessment was based on (2) the inner cell mass (ICM) score or quality, defined in a range from A to C (A: good—prominent, easily noticeable, composed of many cells that form compact and tightly bound structure; B: fair—easily noticeable, many numbers of the cell but are loosely held together; C: poor—difficult to notice, comprising few cells), and (3) the TE score or quality, ranging from A to C (A: good—many cells collectively forming a cohesive, tightly knit epithelium layer; B: fair—few cells, therefore, forming a loose epithelium layer, and C: poor—very few numbers of cells). Based on these three parameters, a standard alphanumeric rating was assigned. Accordingly, a “top-quality” blastocyst was defined as an expanded or hatched blastocyst (score 3 or 4), with both the ICM and TE having at least a fair score (BB). Therefore, all blastocysts of grade ≥3BB were considered top-quality. The blastocysts were cryopreserved using a vitrification protocol (Cryotech, Repro Life Co. Ltd.). The top-quality blastocysts were subjected to FET using a Warming Kit 102 as per the manufacturer’s instructions (Cryotech, Repro Life Co. Ltd.). Embryo quality was scored after thawing and before embryo transfer.
4. Outcomes
The main outcome of this study was a comparison of the usable blastocyst development rate between the COS regimens with GnRHa and CC+hMG. Only usable blastocysts, defined as 3BB or better on day 5 or 6 of culture, were included in the calculation and selected for cryopreservation. Other parameters, such as the duration of stimulation, the total dose of gonadotropins, and the number of oocytes retrieved, were also compared. The secondary outcomes of oocyte and embryo quality between the two groups were the fertilization, clinical pregnancy, miscarriage, and live birth rates. A clinical pregnancy was defined as the detection of a gestational sac on ultrasound. A clinical miscarriage was a case with a documented loss of fetal cardiac activity in an intrauterine pregnancy, loss of a gestational sac, or lack of development of an embryo after at least 7 days. A live birth was defined as a viable infant born after 24 weaks of gestation.
5. Statistical analysis
Outcome measures between the groups were compared using Mann-Whitney U test, Kruskal-Wallis test, and Fisher exact test as appropriate, using the Easy R (EZR) statistical analysis software [16].
Results
1. Study population
AMH is a useful endocrine marker for assessing ovarian reserve. Therefore, based on serum AMH levels all the patients enrolled in this study were grouped into two COS regimens. In total, 95 patients (80.5%; 118 cycles) with serum AMH levels >1.0 ng/mL underwent COS with GnRHa, whereas 23 patients (19.5%; 35 cycles) with serum AMH levels ≤1.0 ng/mL were stimulated with the CC+hMG protocol. There was a 4.1-fold higher number of patients recruited for COS with GnRHa than for COS with CC+hMG.
2. Baseline characteristics
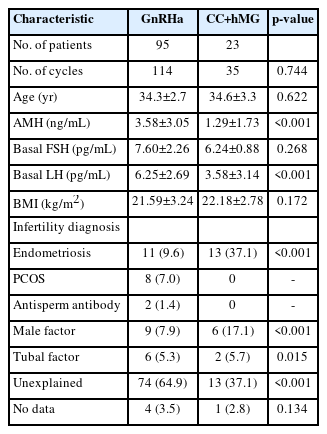
The baseline characteristics of the sample population are depicted in Table 1. A significant difference in the serum AMH levels between the two groups (3.58±3.05 ng/mL vs. 1.29±1.73 ng/mL, p<0.001) was noted (Table 1). The indications for IVF/ICSI–embryo transfer were a tubal factor (5.1%), male factor (8.5%), polycystic ovary syndrome (6.8%), and antisperm antibody (0.8%). The majority of the women undergoing infertility treatment (n=76) were diagnosed with unexplained infertility. Out of the 149 IVF cycles, 87 (58.4%) were performed in patients diagnosed with unexplained infertility. Sixteen women (13.5%) diagnosed with endometriosis underwent 11 and 13 IVF cycles of the GnRHa and CC+hMG regimens, respectively. The body mass index of patients in both groups was comparable (21.6±3.2 kg/m2 vs. 22.2±2.8 kg/m2), without a statistically significant difference (p=0.172). The mean age of patients in the CC+hMG group (34.6±3.3 years) was comparable to that of patients in the GnRHa group (34.3±2.7 years), and there was no statistically significant difference in the mean maternal age (p<0.622) between the groups (Table 1).
3. COS parameters
Both COS regimens used gonadotropins, and the average gonadotropin dosage utilized by patients in the GnRHa group was more than 2-fold that of the CC+hMG group (4,664.0±1,377.4 IU vs. 2,159.6±1,182.7 IU, p<0.001). Similarly, there was a statistically significant difference in the duration of gonadotropin injections (14.8±2.8 days vs. 10.8±3.8 days) between the groups (p<0.001) (Table 2). At the time of the hCG trigger, serum P4 and E2 levels were measured. The mean serum P4 level on the day of the hCG trigger was higher in the GnRHa group (2.54±1.56 ng/mL) than in the CC+hMG group (0.75±0.38 ng/mL); however, these results did not reach statistical significance (p=0.041). Similarly, an approximately four-fold higher serum E2 level on the hCG trigger day was noted in the GnRHa group patients than in the CC+hMG group (12,114.56±6,700.17 pg/mL vs. 2,756.98±2,897.21 pg/mL); this difference was statistically significant (p<0.001). A significant difference was also found in endometrial thickness on the hCG triggering day between the groups (11.1±2.7 mm vs. 8.6±2.7 mm, p<0.001) (Table 2).
4. Laboratory Outcomes
As the GnRHa protocol is beneficial for obtaining more oocytes in a single IVF cycle, in our study the GnRHa group showed a statistically significant difference in the total number of oocytes retrieved per cycle as compared to the CC+hMG group (18.9±8.0 vs. 6.8±4.8, p<0.001). Similarly, a statistically significant difference was observed in the number of mature oocytes (MII) between the groups (15.6±7.7 vs. 5.3±4.4, p<0.001). However, the oocyte maturation rate among the groups (82.4%±12.6% vs. 83.8%±18.9%) was comparable and these results did not reach statistical significance (p=0.131). The GnRHa group exhibited more fertilized embryos (12.7±7.2) 18 hours post-insemination than the CC+hMG group (4.1±4.2). A significant difference was also found between the two groups in the total number of fertilized embryos (p<0.001). Although the fertilization rate in the GnRHa group was seemingly higher (81.2%±14.7%) than that in the CC+hMG group (62.7%±37.3%), this difference did not reach statistical significance (p<0.103) (Table 2). The total number of blastocysts that developed until day 6 of culture in the GnRHa group was significantly higher than in the CC+hMG group (6.4±5.0 vs. 2.8±3.5, p<0.001). However, the blastocyst development rate was comparable between the COS regimens (60.3%±25.9% vs. 50.4%±45.0%), and the difference did not reach statistical significance (p=0.334). Similarly, we calculated the number of top-quality blastocysts (days 5 and 6) in both groups. The average number of top-quality blastocysts was significantly higher in the GnRHa group than in the CC+hMG group (4.4±4.1 vs. 2.0±2.7, p<0.001). However, the main outcome of this study, the top-quality blastocyst development rate (42.7%±24.5% vs. 36.4%±39.1%, p=0.105) remained comparable (Table 2).
5. Clinical and neonatal outcomes
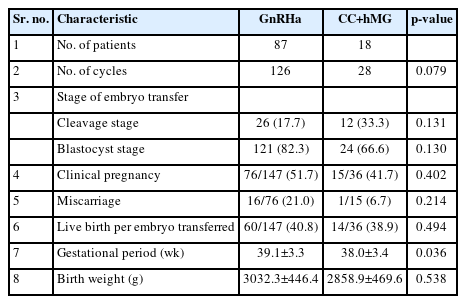
In total, 105 patients (154 cycles) underwent FET. Out of the 154 FET cycles, 126 (81.8 %) used embryos derived from previous GnRHa-based COS cycles, whereas 28 (18.2%) cycles had embryos from previous cycles that used CC+hMG stimulation (Table 3). No significant difference was found between the cleavage and blastocyst stage FET cycles between both groups (p=0.131 and p=0.130, respectively). As secondary outcomes of the study, the patients in the CC+hMG group had lower clinical pregnancy (51.7% vs. 41.7%, p=0.402), miscarriage (21.0% vs. 6.7%, p=0.214), and live birth rates (40.8% vs. 38.9%, p=0.494) than those in the GnRHa group (Table 3). These differences, however, were not statistically significant. In addition to IVF outcomes, in the present study, we assessed the effect of COS on neonatal outcomes, such as gestational period and birth weight. No statistically significant differences were observed between the groups in the gestational period (38.8±3.2 weaks vs. 37.9±2.9 weaks, p=0.036) and birth weight (3,032.3±446.4 g vs. 2,858.9±469.6 g, p=0.036) (Table 3).
Discussion
The results of our study using data from 149 IVF cycles suggest that COS protocols (GnRHa and CC along with hMG) might be associated with blastulation and top-quality blastocyst development rates, although the observed trends did not reach statistical significance. Both COS protocols were comparable in terms of clinical outcomes (clinical pregnancy rate, miscarriage rate, and live birth rate) and neonatal outcomes (gestational period and birth weight) after FET. CC along with gonadotropins has been used in a minimal stimulation protocol, especially in high responders and patients with immature ovarian insufficiency, advanced reproductive age, and low serum AMH levels. However, a previous study reported that the administration of higher doses of gonadotropins for COS in patients with a decreased ovarian reserve and advanced maternal age had no beneficial effect on IVF success, because only a few primordial follicles would be stimulated in each menstrual cycle [17]. Therefore, a lower dosage of gonadotropins was used in the CC+hMG group for COS than in the GnRHa group. One may object that the higher doses of gonadotropins in the GnRHa protocol may negatively affect the implantation potential of the embryo; however, this possibility was ruled out by the use of FET. Similarly, Kol et al. [18] showed that high doses of GnRHa during ovarian stimulation did not influence the implantation potential of embryos in FET cycles.
In each IVF cycle, serum P4 and E2 levels were measured on the day of the ovulation trigger. In our study, higher serum P4 levels were observed on the day of ovulation trigger in the GnRHa group patients than in the CC+hMG group. However, there were no significant differences between the groups. There is some evidence suggesting that elevated P4 levels have (1) a detrimental effect on endometrial receptivity, which could be typically managed with a freeze-all strategy rather than fresh embryo transfer [19], and (2) reduce top-quality blastocyst development [20,21]. Similarly, in our study, the higher P4 levels noted in GnRHa group patients on the day of ovulation trigger resulted in lower blastulation and top-quality blastocyst development rates than in the CC+hMG group. However, this result again did not reach statistical significance. Some contradictory findings have reported comparable top-quality blastocyst development rates between cycles with or without premature P4 elevation [22,23]. In our study, E2 levels were also significantly higher in the GnRHa group than in the CC+hMG group. Several studies have assessed the effect of higher levels of E2 on hCG trigger day on blastulation and top-quality blastocyst development rates. The results of these studies are heterogeneous, and most of the studies included cleavage stage (day 3) embryos. A prospective study with data from 207 IVF cycles found that higher E2 levels (<2,446 pg/mL) had a beneficial effect on embryo quality. However, extremely high levels (>2,446 pg/mL) exhibited adverse effects [24].
In the present study, despite the higher number of retrieved and mature oocytes in the GnRHa group, blastocyst development rates were comparable between both COS regimens. These results again highlight the previously established notion that a higher oocyte yield is not associated with an improved blastocyst development rate [25]. Aggressive ovarian stimulation poses a serious risk of developing OHSS. However, previous studies have confirmed that using GnRHa significantly reduces the risk of developing severe OHSS [26,27]. The incidence of OHSS further significantly decreased when using CC-based mild stimulation regimens [28]. Interestingly, we observed no cases of severe OHSS in either group of patients. Hence, the IVF doctors at our clinic have addressed this key issue by creating an OHSS-free IVF program, which is the objective of many IVF units worldwide. CC-based mild stimulation protocols are associated with a higher rate of cycle cancellation. However, in a cohort of 149 IVF cycles, only three cycles were canceled and excluded at the earliest step of cycle selection. It is a well-recognized fact that the abnormal maternal hormonal milieu produced by COS including CC may negatively affect endometrium receptivity and thereby pregnancy outcomes during fresh autologous IVF cycles; therefore, in GnRHa- and CC+hMG-based ovarian stimulation cycles, FET is recommended [29,30]. The pregnancy outcomes (clinical pregnancy, miscarriage, and live birth rates) among the study groups were comparable. Previous studies have suggested that ovarian stimulation protocols are associated with adverse neonatal outcomes such as low birth weight and risk of preterm delivery in fresh embryo transfer cycles [12]. However, in our study using FET, the neonatal outcomes (gestational period and birth weight) were comparable among both COS regimens. Only three pregnancies in the CC+hMG group resulted in preterm (<37 weaks) deliveries. Interestingly, none of the newborns in either group reported very low birth weight (<1,500 g).
Comparative analyses of the present study suggest that COS regimens with GnRHa and CC along with gonadotropins may not affect the top-quality blastocyst development rate. Following FET in subsequent cycles, the IVF outcomes, such as the clinical pregnancy, miscarriage, and live birth rates, remained unaffected. Previous studies have suggested that as compared to natural cycles, COS using CC along with hMG in cycles with fresh embryo transfers were associated with the highest proportion of small for gestational age in the entire cohort. That protocol also showed the highest adjusted odds ratio (AOR) for low for birth weight (<2,500 g) (AOR, 1.67; 95% confidence interval [CI], 1.45 to 1.73), very low for birth weight (<1,500 g) (AOR, 2.38; 95% CI, 1.52 to 3.72), and small for gestational age (AOR, 1.71; 95% CI, 1.47 to 1.98). Moreover, the protocol was significantly associated with a higher incidence of cesarean section deliveries [12]. In the present study, unlike fresh embryo transfer cycles, perinatal outcomes such as birth weight and gestational length were not associated with the prior COS regimen. Furthermore, large population studies, including randomized controlled trials, are needed to investigate and the effect on perinatal outcomes. However, the interpretation of the findings of this study is limited by the overall sample size, the distribution of sample sizes between the groups, and the possibility of selection and confounding biases. In the present study, we did not attempt to investigate the association of infertility diagnoses with blastocyst development and IVF or neonatal outcomes. The effect of different infertility diagnoses on pre-implantation embryo development, clinical, and perinatal outcomes after IVF is a new area of future research.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Author contributions
Conceptualization: SAB. Data curation: HO. Formal analysis: SAB, KN. Methodology: SAB, TI, KK. Project administration: KN, HO, KK. Visualization: SAB, TI. Writing-original draft: SAB. Writing-review & editing: SAB, KN, HO, TI, KK.
Acknowledgements
The authors acknowledge the Kinoshita Ladies Clinic staff and patients for their assistance in this project. The anonymous reviewers are greatly acknowledged.