Human sperm parameter improvement associated with Ceratonia siliqua extract as a cryopreservation supplement after vitrification
Article information
Abstract
Objective
Given the destructive effects of oxidative stress on sperm structure, this study was conducted to investigate the antioxidant effects of different concentrations of Ceratonia siliqua plant extract on human sperm parameters after the freezing-thawing process.
Methods
A total of 20 normozoospermic samples were frozen. Each sample was divided into two control groups (fresh and cryopreservation) and three cryopreservation experimental groups (containing C. siliqua extract at concentrations of 20, 30, and 40 µg/mL in the freezing extender). Motility, intracellular levels of reactive oxygen species (ROS), plasma membrane integrity (PMI), mitochondrial membrane potential (MMP), viability, and acrosome reaction parameters were evaluated.
Results
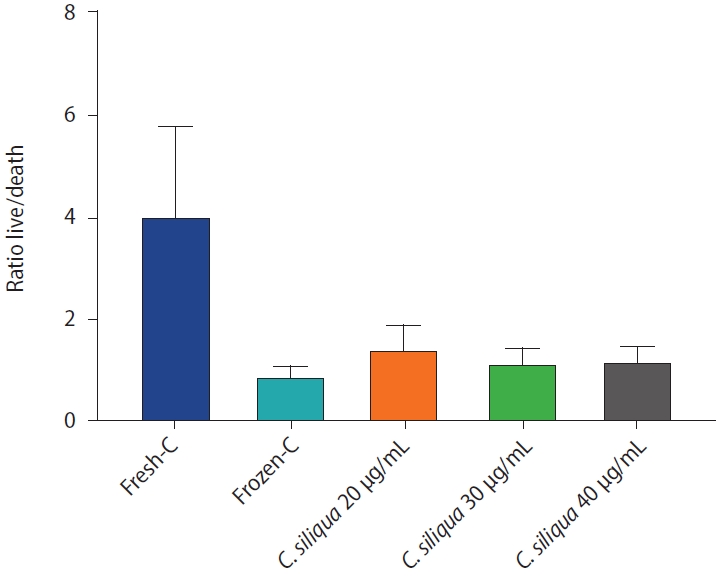
Statistical analysis showed that the highest motility, viability, and PMI were associated with the 20 µg/mL concentration of C. siliqua extract. At all concentrations, intracellular ROS levels were significantly lower and the levels of MMP and the acrosome reaction were significantly higher than in the cryopreservation control group (p≤0.05).
Conclusion
C. siliqua extract supplements at concentrations of 20, 30, and 40 µg/mL improved sperm motility, viability, PMI, MMP, intracellular ROS, and the acrosome reaction.
Introduction
Freezing human sperm is an effective and beneficial strategy in the field of male fertility [1]. This method can be used for men with cancer who are undergoing radiotherapy or chemotherapy treatment, in sperm donation programs, or in conjunction with surgical procedures that endanger male fertility [2-5]. The use of cryopreservation to create a sperm bank for healthy men who are exposed to ionizing radiation, biological contaminants, or toxins at work is another related goal [6]. Despite these benefits of sperm cryopreservation, we now understand that cryopreservation and thawing cause irrecoverable changes in sperm function and structure [7]. In the cryopreservation process, sperm are exposed to stressors such as osmotic pressure change, pH change, dehydration, the creation of ice crystals, and the generation of free radicals [8]. These factors can impair sperm motility, cell membrane and mitochondrial structure, chromatin structure, and sperm viability [9]. In recent years, extensive efforts have been made to improve cryopreservation and reduce the harmful effects of the freezing-thawing process on human sperm [10]. These include changes to cryopreservation methods, composition of the cryopreservation medium, cryopreservation and thawing times, and packaging of samples [11,12]. In general, freezing media include cryoprotectants, ionic or non-ionic materials to maintain the pH, and an energy substrate, as well as fatty acids, antibiotics, and antioxidants [11]. According to previous studies, the complementation of freezing-thawing environments with various factors, including antioxidants, is an effective approach to improve the quality of frozen-thawed sperm [13]. Many studies have focused on plants with antioxidant capacities that can counteract the damaging effects of free radicals due to oxidative stress [14]. Ceratonia siliqua is an evergreen plant native to the Mediterranean region. C. siliqua contains compounds such as vitamins (B, C, D, and E), polyphenols, and minerals (iron, phosphorus, potassium, sodium, and calcium) [15,16]. The phenolic components of this plant improve oxidative stress conditions [17] and can act as a powerful source of antioxidants. As such, the use of phenolic antioxidants is recommended to improve oxidative damage, with minimal side effects and ease of use [12,18]. For the first time, in the current study, we simultaneously evaluated the effects of C. siliqua extract on human sperm parameters including motility, viability, cell membrane and mitochondrial potential, intracellular levels of reactive oxygen species (ROS), and the acrosome reaction using computer-assisted sperm analysis, fluorescence, hypoosmotic swelling (HOS) testing, JC-1 staining, flow cytometry, and fluorescent thiocyanate, respectively.
Methods
1. Extraction
1) Preparation of C. siliqua hydroalcoholic extract
The C. siliqua plant was prepared by a medicinal plant expert from the local plant market and approved by a pharmacognosy expert. First, 100 g of fresh C. siliqua plant matter was dried at room temperature and then ground in a blender, yielding a homogeneous powder. Then, 10 g of C. siliqua powder was mixed with 1,000 mL of 96% ethanol (Merck). The resulting solution was added to distilled water in a 50:50 ratio and kept at room temperature for 72 hours. This extract was then filtered (filter paper no. 4; Whatman PLC). The filtered solution was concentrated at 50 °C using a rotary apparatus. Finally, a brown extract was obtained, dried in an oven at 40 °C, and kept at −20 °C until use [19].
2. Participation and semen collection
This study protocol was approved by the ethics committee of the Hamadan Branch of Azad University. A total of 20 normal sperm samples from men referred to the Aban Infertility Center between April 29, 2021 and August 17, 2021 were used. Samples were prepared during 3 to 5 days of abstinence. These normal samples had normal morphology (>4%), normal motility (<40%), and a concentration above 15 million/mL and were collected according to the 2010 World Health Organization guidelines.
3. Sperm processing
The swim-up method was used to process the semen samples [20]. In this method, the samples were first centrifuged for 5 minutes at 300 ×g; then, the supernatant was removed, and the pellets were precipitated with preheated human tubal fluid (HTF) and 2.5% human serum albumin (HSA) (Vitrolife) [21]. These samples were poured into a Falcon tube and incubated at a 45° angle in an incubator (5% CO2, 37 °C) for 1 hour. The supernatant was poured into a microtube, analyzed, and then frozen by vitrification.
4. Cryopreservation and thawing
After the preparation of sperm, the samples were frozen via the microdroplet technique [22]. For the cryopreservation control group, the sperm solution and HTF were mixed with a solution containing 5% HSA and 0.5 mol/L sucrose (Vitrolife) at a 1:1 ratio. C. siliqua extract was prepared at several concentrations (20, 30, and 40 µg/mL). For the cryopreservation experimental groups, the sperm solution was mixed with HSA and sucrose solution along with 20, 30, or 40 µg/mL of C. siliqua extract.
Droplets of 30 µL of the micropipette-prepared suspensions were poured into a metal strainer and placed in liquid nitrogen, and the frozen samples were kept in nitrogen storage tanks for 1 week [23]. For the thawing process, we first incubated the HTF medium for 2 hours at 37 °C and then immersed the frozen samples in 5 mL of heated HTF supplemented with 1% HSA. Next, the sperm suspensions were incubated at 37 °C and 5% CO2 for 5 minutes. Finally, the samples were centrifuged for 5 minutes at 1,800 rpm, the pellets were resuspended in 50 µL HTF, and the sperm were evaluated [23].
5. Assessment of sperm parameters
1) Motion characteristics
A computer-aided sperm analyzer system (Sperm Class Analyzer version 6; Microptic) was used to assess motility and the motility indices. In this study, 5-µL samples from each group were placed in a preheated Makler chamber (Proiser), and general motility, progressive movement and motor indices, average path velocity (μm/sec), curvilinear velocity (μm/sec), straight-line velocity (μm/sec), mean linearity (μm/sec), straightness (μm/sec), amplitude of lateral head displacement (μm), and beat cross frequency (Hz) were assessed for 500 sperm.
2) Viability
To assess sperm viability, a LIVE/DEAD sperm kit was used according to the manufacturer’s instructions (L-7011; Molecular Probes). To accomplish this, 0.1 µL of SYBR14 working solution (containing 150 mM sodium chloride, 10 mM HEPES, 10% bovine serum albumin, and SYBR14) was added to 50 µL of sperm suspension. The resulting solution was incubated at 37 °C for 10 minutes. Then, 1 µL of propidium iodide solution was added to the previous suspension and incubated for another 5 minutes in an incubator at 37 °C, and 10 µL of Hancock solution was added to the solution to immobilize the sperm. Finally, to assess viability, 6 µL of this final suspension was placed on a glass slide, covered, and examined using a fluorescence microscope (CX21; Olympus; excitation at 450–490 nm, emission at 520 nm) with a magnification of ×1,000. In this protocol, cells were stained with propidium iodide and SYBR14 on each slide. Dead and living spermatozoa were evaluated based on red and green color emissions, respectively. The LIVE/DEAD sperm ratio is depicted in Figures 1 and 2 [21].
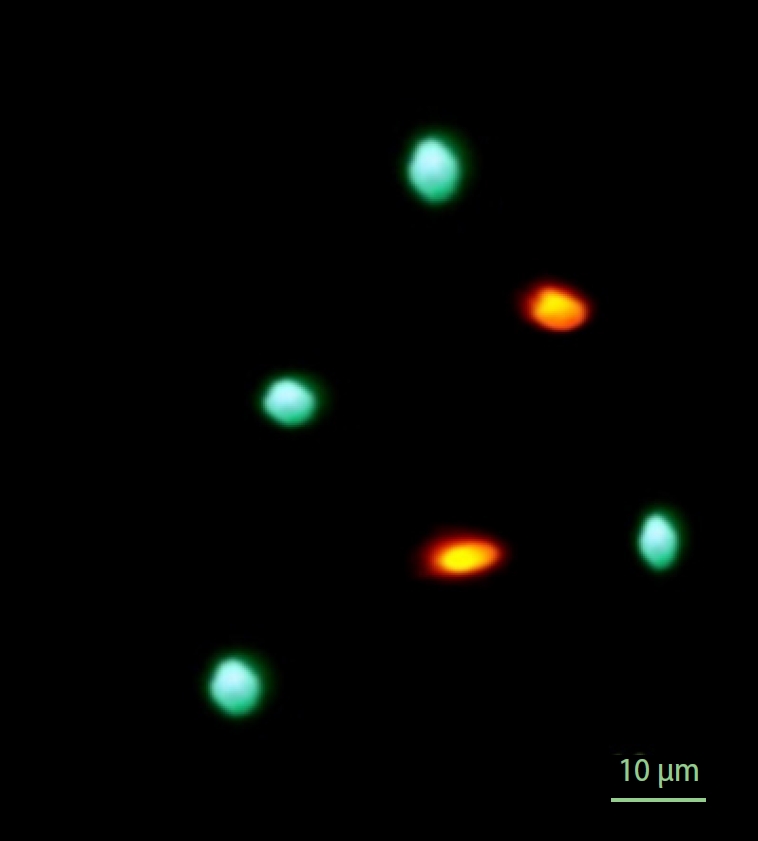
This shows dead and live sperm based on the fluorescence emitted. Green sperm stained with SYBR14 are alive and orange sperm stained with propidium iodide are dead.
3) Plasma membrane integrity
The HOS test was used to evaluate membrane integrity. We mixed 10 µL of the sperm sample, 100 µL of HOS solution containing 0.73 g of sodium citrate and 1.35 g of fructose (Merck), and 100 mL of distilled water and incubated the mixture (5% CO2, 37 °C). Then, 15 µL of this solution was added to 5 µL of eosin Y solution (2%), and a smear was prepared from the resulting mixture on a glass slide. Finally, 200 sperm were assessed using a light microscope (ECLIPSE 50i; Nikon) with a magnification of ×1,000. Coiled-tail sperm were considered to have intact plasma membranes (Figure 3) [24].
4) Mitochondrial activity
JC-1 is a lipophilic cationic dye (T4069; Sigma-Aldrich) used to assess sperm mitochondrial activity. For this assessment, sperm samples were centrifuged for 5 minutes at 500 ×g, after which the pellet was diluted with phosphate-buffered saline. Then, 500 µL of this solution was mixed with 1 µL of JC-1 stock solution and incubated at 37 °C for 40 minutes. Next, 10 µL of Hancock solution was added to the previous solution to immobilize the sperm. Finally, 2.5 µL of the prepared sample was placed on a glass slide, covered, and assessed under a BX51 fluorescence microscope (Olympus; excitation at 450–490 nm, emission at 520 nm) at a magnification of ×1,000, under which 200 sperm per slide were examined. Sperm with yellow/orange fluorescence at the midline were considered to display high mitochondrial activity, while sperm with green fluorescence were considered to exhibit low mitochondrial activity (Figure 4) [25].
5) Acrosome integrity
To evaluate the integrity of the acrosome, fluorescein isothiocyanate-conjugated Pisum sativum agglutinin (FITC-PSA, L0770; Sigma-Aldrich) was used. Initially, a 30-µL smear was prepared on a glass slide for each sample. After drying, the samples were fixed at room temperature with methanol for 30 minutes. Then, 50 µL of FITC-PSA solution was poured on each slide and incubated for 30 minutes. Finally, the stained slides were washed with distilled water and were evaluated using a BX51 fluorescence microscope (Olympus; excitation at 450–490 nm, emission at 520 nm) with a magnification of ×1,000. For each slide, 200 sperm were examined. Sperm with bright green fluorescence in the acrosome area were identified as having intact acrosomes, while sperm with no green or pale green fluorescence near the equator were identified as acrosome-reacted (Figure 5) [21].
6) Intracellular ROS
To assess intracellular ROS, sperm samples were first suspended after melting with phosphate-buffered saline. Then, 10 µL of dihydroethidium solution was added. The samples were incubated at room temperature for 30 minutes. For this measurement, flow cytometry was used. Red fluorescence with an FL2 detector (525–625 nm) was used to indicate intracellular ROS [26,27].
7) Flowcytometric analysis
Flowcytometric analysis was conducted using Cyflogic version 2.5.1 (CyFlo Ltd.). This analysis was performed using a FACSCalibur flow cytometer (BD Biosciences) and an argon laser with a wavelength of 488 nm [28].
Results
As shown in Table 1, when either 30 or 40 µg/mL of C. siliqua extract was added to the sperm freezing extender, no significant differences in total or progressive motility were observed relative to the cryopreservation control group. In contrast, significantly greater motility was observed in the group treated with 20 µg/mL extract (p≤0.05). No significant difference was observed in the other motility characteristics for any of the extract concentrations relative to the cryopreservation control group (Table 1).

Effect of Ceratonia siliqua concentrations 20, 30, and 40 μg/mL added to cryopreservation/thawing media on motility parameters of human spermatozoa
As shown in Table 2, the intracellular ROS level was significantly reduced among sperm treated with any concentration of C. siliqua extract relative to the cryopreservation control group (p≤0.05). Additionally, by increasing the concentration of C. siliqua extract from 20 µg/mL to 30 or 40 µg/mL, viability and plasma membrane integrity (PMI) were significantly improved in the cryopreservation experimental groups relative to the cryopreservation control group. The greatest increase relative to the control was associated with the 20 µg/mL extract concentration, and no significant difference was observed between 30 and 40 µg/mL (p≥0.05). Levels of both mitochondrial membrane potential (MMP) and the acrosome reaction showed a significant increase at all three concentrations compared to the cryopreservation control group. However, no significant difference was seen between the experimental groups (Table 2).
Discussion
Sperm cryopreservation can involve the formation of ice crystals, osmotic imbalance, and oxidative stress, potentially causing irreparable damage to the structure and ultimately the function of the cell [29]. One method to prevent the formation of ice crystals and the associated damage is cryopreservation by vitrification [30]. In this process, sperm cryopreservation is performed more quickly and with greater safety than in other methods [31,32]. The cryopreservation process plays a role in the production of ROS by accelerating the conversion of anion superoxide to hydrogen peroxide [33]. Although a moderate ROS level is required for optimal sperm function, high levels are associated with the impairment of function [34]. ROS produced as a result of cryopreservation can damage the lipid, protein, and DNA structures of the sperm [35].
Studies have shown that cryopreservation reduces the activity of the sperm antioxidant system, leading to impaired cell motility, integrity, and membrane fluidity [33]. C. siliqua extract can protect against oxidative stress [15,36]. Various studies have indicated that the protective effect of this plant extract is due to its strong antioxidant properties [12,19,37]. The results of the current study confirm the protective properties of this extract against oxidative stress, as the intracellular ROS level was significantly reduced at all concentrations of C. siliqua extract. Although no previous research had directly examined the intracellular ROS level in sperm treated with C. siliqua extract, the improvement of ROS-dependent parameters in other studies aligns with this result. Previous studies have shown that C. siliqua extract can increase the activity of antioxidant enzymes such as catalase and superoxide dismutase [15], so the reduction of intracellular ROS during cryopreservation can be explained by the capacity of C. siliqua extract to stimulate these enzymes.
Notably, mitochondrial activity in oxidative phosphorylation can be a source of ROS production [38]. At all three concentrations of C. siliqua extract, the sperm MMP was significantly increased relative to the cryopreservation control group, most likely by reducing the level of intracellular ROS. Motility is an essential sperm parameter that can have a positive or negative effect on fertility. In the current study, the total and progressive movement in the cryopreservation control group were significantly lower than in the fresh group, likely due to the sensitivity of the adenosine triphosphate -dependent sodium-potassium pump and the consequent leakage of ions related to movement [39]. In the sperm treated with the extract, the total and progressive movement were improved compared to the cryopreservation control group, most notably in the sperm treated with 20 µg/mL of the extract. These findings align with the results of Sabzeie et al. [19] and Faramarzi et al. [12]. Like the present study, those studies revealed not only a positive effect of C. siliqua extract on motility, but also an optimal extract concentration of 20 µg/mL. This is likely because C. siliqua extract has a greater capacity to reduce superoxide anions at 20 µg/mL than at other concentrations. However, for the other motor characteristics (average path velocity, curvilinear velocity, straight-line velocity, mean linearity, straightness, amplitude of lateral head displacement, and beat cross frequency), we found no significant differences between the concentrations of 20, 30, and 40 µg/mL or in comparison with the cryopreservation control group, which contradicts the study of Sabzeie et al. [19]. Motility is a known indicator of survival and of other health parameters such as PMI, mitochondria health, and even sperm DNA damage. Our results showed that the presence of C. siliqua extract in cryopreservation can help increase sperm viability. This finding supports the results of the Faramarzi et al. [12] study, which revealed a higher percentage of sperm survival in groups treated with the extract than in the control group; additionally, the survival rate of sperm treated with 20 µg/mL was significantly higher than among the control group or the sperm treated with 10 or 40 µg/mL. The improvement of this parameter can likely be attributed to the antioxidant contents of gallic acid, chlorogenic acid, cinnamic acid, and caffeic acid in C. siliqua extract. Generally, these compounds have been shown to reduce intracellular ROS and increase sperm viability. In the current study, the results of the HOS test indicated that the greatest membrane integrity was present in the group treated with 20 µg/mL of extract, while this parameter was not significantly different in the groups with concentrations of 30 and 40 µg/mL. This finding is completely consistent with the result of Sabzeie et al. [19]. In our study, the presence of C. siliqua extract at all concentrations (20, 30, and 40 µg/mL) led to a significant increase in the acrosome reaction compared to the cryopreservation control group. This result can be attributed to the antioxidant roles of this extract in countering the damage caused by oxidative stress as well as in osmotic balance regulation, membrane preservation, conjugation, and calcium regulation. Accordingly, the role of extracellular antioxidants in improving sperm membrane resistance can be explained by the reduction of lipid peroxidation [40].
In conclusion, C. siliqua extract supplements at concentrations of 20, 30, and 40 µg/mL improve sperm motility, viability, cell membrane and mitochondrial potential, intracellular ROS, and the acrosome reaction. A concentration of 20 µg/mL has a particularly high capacity to reduce intracellular ROS, oxidative stress, and its products, eventually improving ROS-affected parameters such as motility, PMI, MMP, and the acrosome reaction.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Author contributions
Conceptualization: YK, ES. Data curation: FM. Formal analysis: TF. Funding acquisition: ES. Methodology: YK. Project administration: TF. Visualization: TF. Writing-original draft: YK. Writing-review & editing: FM, YK.