Seminal prolactin is associated with HSP90 transcript content in ejaculated spermatozoa
Article information
Abstract
Objective
Evidence indicates that an imbalance between the production of reactive oxygen species and defense ability of antioxidants has clinical significance in the pathophysiology of male infertility. To investigate the role of seminal prolactin (PRL) in the fertilizing capacity of men, the present study evaluated the associations of seminal PRL levels with semen parameters and heat shock protein 90 (HSP90) transcript abundance in ejaculated spermatozoa.
Methods
We assessed seminal PRL levels and the abundance of HSP90 transcripts in ejaculated spermatozoa from normozoospermic donors (n=18) and infertile men (n=18). The transcript content of HSP90 in ejaculated spermatozoa was analyzed using real-time polymerase chain reaction.
Results
Seminal PRL concentrations in infertile patients were significantly lower (p=0.004) than in fertile controls. Seminal PRL showed relatively good diagnostic power for discriminating infertile men (area under the curve=0.776; 95% confidence interval, 0.568 to 0.934; p=0.005). Significant positive correlations were seen between seminal PRL levels and sperm count (r=0.400, p=0.016) and progressive motility (r=0.422, p=0.010). Infertile patients showed a significantly higher abundance of sperm HSP90 than fertile controls (p=0.040). Sperm HSP90 transcript abundance was negatively correlated with sperm progressive motility (r=0.394, p=0.018). Men with higher seminal PRL levels exhibited a lower abundance of sperm HSP90 transcripts.
Conclusion
Our finding demonstrated associations among semen quality, seminal PRL levels, and the abundance of HSP90 transcripts in ejaculated spermatozoa. Seminal PRL may contribute to male fertility by maintaining the seminal antioxidant capacity and may have the potential to act as a diagnostic and prognostic biomarker.
Introduction
Infertility is a public health concern with psychological, social, emotional, and financial implications that is estimated to affect 8% to 12% of reproductive-age couples worldwide [1]. It has been reported that a male factor contributes to infertility in approximately 50% of couples who fail to conceive [2]. Evidence shows that oxidative stress—an imbalance between reactive oxygen species (ROS) production and antioxidant defense ability—has clinical significance in the pathophysiology of male infertility [3]. Excessive generation of ROS and/or a decreased available antioxidant defense system leads to abnormal sperm function by affecting the plasma membrane and DNA integrity [4]. There are various enzymatic and non-enzymatic antioxidants in the seminal plasma that are essential for protecting spermatozoa from oxidative damage [5].
Prolactin (PRL), a 198-amino-acid polypeptide hormone, is synthesized by lactotroph cells and secreted from the anterior pituitary in a pulsatile manner. It weighs 23 kDa, and its secretion is affected by many factors, including stress, sleep, and food [6]. It has been shown that human PRL can increase the viability of breast cancer cells treated with DNA-damaging agents [7]. Receptors of PRL have been identified on spermatogenic, Leydig, and Sertoli cells, as well as on efferent duct epithelial cells, suggesting a potential role for this hormone in promoting secretory/adsorptive functions, steroidogenesis, and spermatogenesis in the male reproductive system [8].
In addition, PRL is present in human seminal plasma [9] and has been shown to affect sperm metabolism [10] and motility [11,12]. It also exerts a prosurvival effect on spermatozoa cells mediated by the suppression of apoptosis, inhibits DNA strand breaks, and suppresses their entry into a state of capacitation [13]. Moreover, the potential antioxidant activity of PRL in several cells and tissues has been suggested. Research has shown that PRL significantly reduces total lipid peroxidation (LPO) product levels in rat hippocampal neurons and prevents glutamate-induced mitochondrial dysfunction [14]. A significant increase in both the mRNA and protein of superoxide dismutase 1 (SOD1) and SOD2 in luteinized granulosa cells exposed to PRL has been reported [15]. Evidence also exists that PRL prevents hydrogen peroxide-induced damage to human retinal pigment epithelial cells by reducing intracellular levels of ROS [16].
Heat shock proteins (HSPs) are described as chaperones with protective and anti-apoptotic roles in cells that are induced in response to both intrinsic and extrinsic stressors [17]. HSP90, the most abundant cytoplasmic chaperone, is upregulated in response to increased levels of free radicals and protects cells against oxidative stress [18]. HSP90 protects metastable regulatory molecules such as kinases and steroid hormone receptors and supports the maturation and folding of newly synthesized proteins [19]. Higher levels of sperm HSP90 transcripts have been reported in couples experiencing recurrent idiopathic pregnancy loss [20]. It has also been reported that HSP90 plays an important role in the regulation of sperm motility [21,22].
The maturation or stability of the PRL receptor, as a client protein of HSP90, depends on HSP90, and inhibition of this master chaperone leads to the loss of PRL receptors due to proteolytic degradation [23]. The survival of breast cancer cells and normal mammary epithelial cells was found to be promoted by PRL and HSP90 in a cellular context-dependent manner [24]. HSP90 is involved in PRL-induced apoptosis signaling, and the inhibition of PRL receptors promotes spermatogonial apoptosis during spermatogenesis [25]. Thus, in the present study, we assessed seminal PRL levels and sperm HSP90 transcript content in fertile and infertile men to evaluate the associations of seminal levels of PRL with sperm parameters and HSP90 transcript content.
Methods
1. Subjects
The present study was approved by the Ethics Committee of the Department of Biology, Shahid Chamran University of Ahvaz (EE/97.24.3.70393/scu.ac.ir). All participants provided written informed consent. Normozoospermic volunteers (n=18) as controls and infertile patients (n=18) attending the Narges Medical Genetics and Prenatal Diagnosis Laboratory in Ahvaz, Iran were recruited for semen analysis. The infertile men were individuals with abnormal semen characteristics in at least one parameter according to the 2010 World Health Organization (WHO) guidelines. Smokers, drug users, alcohol consumers, and men with an abnormal body mass index, history of varicocele, cryptorchidism, prostatitis, urinary tract infection, genital trauma, testicular torsion, inguinal or genital surgery, sexually transmitted disease, chronic illness, and serious systemic diseases were excluded from the study.
2. Semen analysis
After 3 days of sexual abstinence, semen samples were collected in sterile containers by masturbation, allowed to liquefy for 30 minutes, and then were analyzed immediately. According to the 2010 WHO guidelines, a concentration of ≥15×106/mL, progressive motility of ≥32%, normal sperm morphology of ≥4% and a leukocyte content of <1×106/mL were considered normal.
3. Assessment of seminal PRL levels
Seminal levels of PRL were measured based on the enzyme immunoassay technique using the AccuBind ELISA Microwells Kit (Monobind Inc.) with an Immunoassay Analyzer Cobas e 411 (Roche Diagnostics GmbH). The enzyme immunoassay was carried out according to the manufacturer’s instructions, and the absorbance of each microwell was read at 450 nm.
4. Sperm RNA extraction
For the elimination of somatic cells, semen samples were treated with cell lysis buffer (0.1% sodium dodecyl sulfate and 0.5% Triton X in DEPC-treated water) after two washes with phosphate-buffered saline (Ca2+Mg2+-free; pH 7.4, 0.1 mM). Then, according to the manufacturer's instructions, TRIzol reagent (Life Technology) was added to each sperm pellet to extract total RNA. After treatment with RNase-free DNase (Qiagen GmbH), the concentration of extracted RNA was determined using a NanoDrop-2000 spectrophotometer (Thermo Fisher Scientific).
5. Real-time reverse-transcription polymerase chain reaction analysis
Total RNA from each sperm pellet was reverse-transcribed into cDNA in a final volume of 10 μL using the PrimeScript RT reagent Kit (TaKaRa) based on the manufacturer’s instructions. Real-time quantitative polymerase chain reaction (PCR) analysis for each prepared cDNA was carried out in triplicate using SYBR Green PCR Master mix (TaKaRa) in an ABI Step One Real Time PCR system (Applied Biosystems). β-Actin was applied as a control gene to normalize the HSP90 mRNA content, and the cycle threshold (ΔCt) value was calculated for each sample.
Oligo primer analysis software version 7.0 (Molecular Biology Insights) was used to design the mRNA primers. The specificity of the primers was verified using the BLAST website (https://www.ncbi.nlm.nih.gov/tools/primer-blast/) (Table 1).
6. Statistical analysis
All statistical analyses were performed in SPSS version 16.0 (SPSS Inc.). The independent-sample t-test was used to compare sperm parameters between fertile and infertile men. The non-parametric Mann-Whitney test was performed to assess differences in seminal PRL and ΔCt of sperm HSP90 mRNA between subjects. Spearman correlation coefficients were used to quantify the relationships between variables. The optimal cutoff value of seminal PRL for diagnosis was determined using receiver operating characteristic (ROC) curve analysis. Differences were considered statistically significant at p-values less than 0.05.
Results
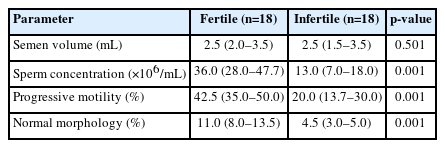
The median and interquartile ranges of seminal parameters in infertile patients and normozoospermic controls are presented in Table 2. The mean age of infertile patients (mean±standard error of the mean [SEM], 34.3±1.50 years [range, 25 to 46]) showed no significant difference (p=0.305) compared to normozoospermic donors (mean±SEM, 36.8±1.86 years [range, 22 to 56]). The sperm concentration differed significantly (p=0.001) between infertile and fertile subjects. A significantly higher (p=0.001) count of sperm with progressive motility was observed in fertile individuals than in infertile men. Moreover, infertile patients had significantly higher (p=0.001) levels of sperm with abnormal morphology (Table 2).
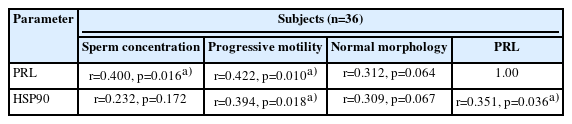
Infertile patients showed significantly lower (p=0.004) seminal PRL levels than normozoospermic controls (Figure 1A). Seminal PRL showed an optimal diagnostic cutoff value of 15.70 ng/mL with 83.30% sensitivity and 66.7% specificity to distinguish between fertile and infertile subjects (Figure 1B). ROC curve analysis demonstrated that seminal PRL, with an area under the curve (AUC) of 0.776 (95% confidence interval [CI], 0.568 to 0.934; p=0.005), had relatively good diagnostic power for discriminating infertile men. Seminal levels of PRL were correlated significantly with sperm concentration (r=0.400, p=0.016) and progressive motility (r=0.422, p=0.010). No significant correlation was seen between seminal PRL levels and sperm morphology (Table 3).
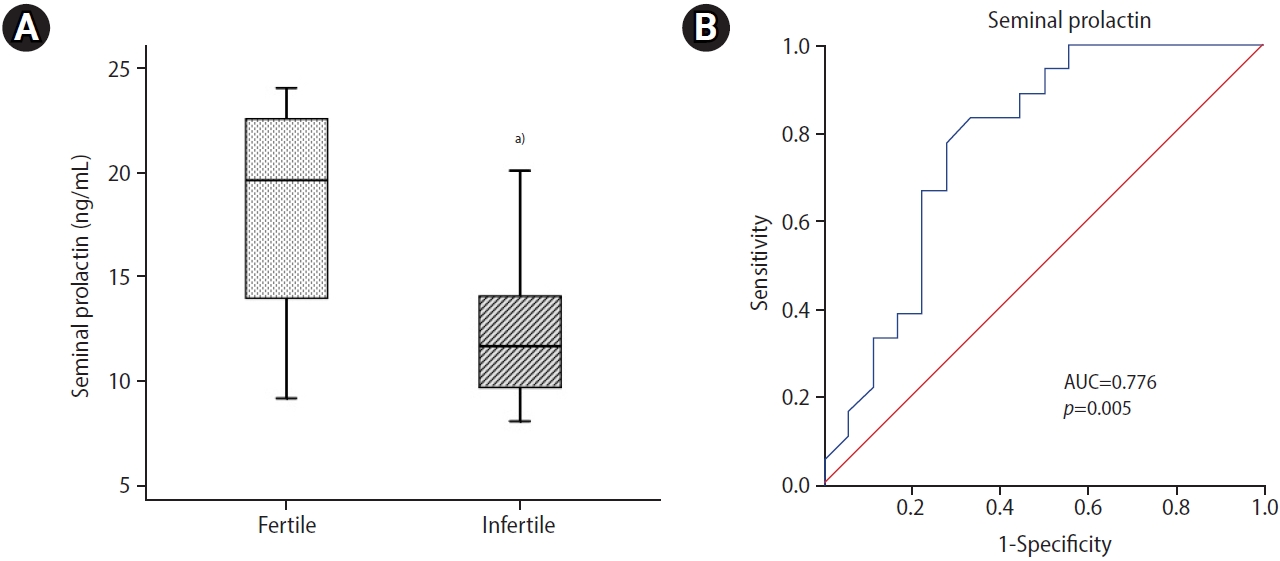
(A) Seminal levels of prolactin (PRL) in infertile patients were significantly lower than in normozoospermic controls (box plot). The Mann-Whitney U test was done as the test of significance. (B) Receiver operating characteristic curve analysis revealed that seminal PRL had a fairly good diagnostic value for distinguishing between fertile and unexplained infertile men, with an area under the curve (AUC) of 0.776 (95% confidence interval, 0.618 to 0.934; p=0.005; cutoff value, 15.7 ng/mL; sensitivity, 83.3%; specificity, 66.7%). a)p=0.004.
In infertile patients, the median sperm HSP90 ΔCt value was significantly lower (p=0.040) than in normozoospermic controls (Table 4). This indicated that there was higher abundance of HSP90 mRNA in infertile men (Figure 2). A significant correlation was observed between sperm HSP90 ΔCt values and sperm progressive motility (r=0.394, p=0.018) (Table 3). Higher sperm HSP90 transcript abundance was significantly associated with reduced sperm progressive motility. Sperm HSP90 transcript abundance showed no significant correlations with sperm concentration or morphology. Furthermore, there was a significant correlation between the transcript content of HSP90 in ejaculated spermatozoa and seminal levels of PRL (r=0.351, p=0.036) (Table 3). Higher levels of seminal PRL were significantly associated with reduced sperm HSP90 transcript content.
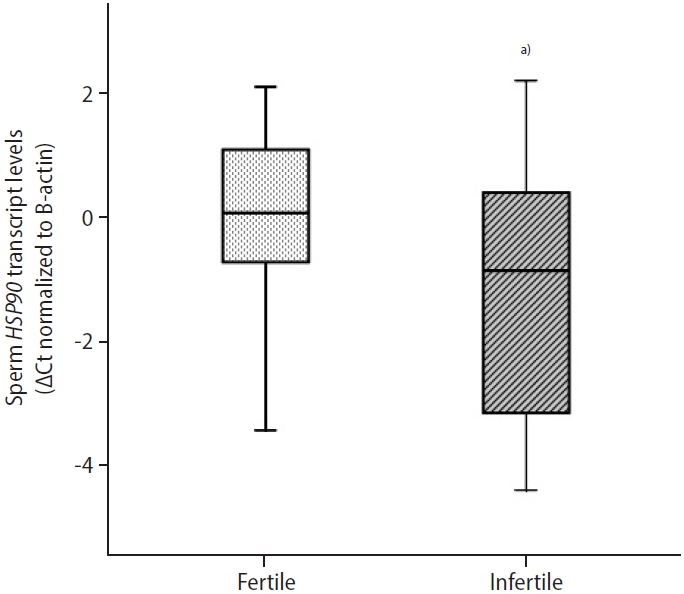
Sperm heat shock protein 90 (HSP90) normalized cycle threshold (Ct) values in infertile patients and normozoospermic controls. HSP90 transcript levels in ejaculated spermatozoa were significantly higher in infertile patients than in normozoospermic men (box plot). Low normalized Ct values indicate high mRNA expression levels. The Mann-Whitney U test was done as the test of significance. a)p=0.040.
Discussion
Seminal plasma is a biofluid rich in different organic and inorganic compounds that are essential for the maintenance of sperm function. Reproductive hormones are among the contents of seminal plasma and may play vital roles in the fertilizing ability of spermatozoa. An analysis of seminal hormones enables the identification of underlying molecular pathways associated with the pathology of male infertility and their potential use as non-invasive biomarkers. There have been reports of a role for seminal PRL in male fertility. The present study showed an association of seminal levels of PRL with HSP90 transcript content in ejaculated spermatozoa. Our findings indicated that the mean concentrations of seminal PRL were 18.07±5.3 and 12.69±3.7 ng/mL in fertile and infertile men, respectively. The seminal levels of PRL in normozoospermic controls were significantly higher than that in infertile patients. Higher levels of seminal PRL have been reported in normozoospermic men than in oligozoospermic patients [26-29]. Tang and Chan [30] reported that the seminal levels of PRL in azoospermic patients were significantly lower than in normozoospermic men. Moreover, Chan et al. [11] found that seminal levels of PRL did not meaningfully differ between normozoospermic and oligozoospermic men, but were significantly lower in azoospermic men. They also reported that seminal PRL concentrations were higher in subjects with higher sperm motility. ROC curve analysis demonstrated that seminal PRL with an AUC of 0.776 (95% CI, 0.568 to 0.934; p=0.005) has relatively good diagnostic power to discriminate infertile men. Seminal PRL levels differentiated infertile subjects from fertile individuals at a cutoff value of 15.70 ng/mL with 83.30% sensitivity and 66.7% specificity.
The present study demonstrated significant correlations between seminal levels of PRL and sperm concentration and motility. Significant associations were previously reported between seminal PRL concentrations and sperm count [31], motility [30,31] and viability [32]. Gonzales et al. [33] demonstrated that seminal PRL concentrations had a significant positive correlation with sperm motility and were related negatively to sperm concentration. However, Chan et al. [11] showed that there was no association between seminal levels of PRL and sperm parameters. Our findings also indicated that there was no correlation between seminal PRL levels and sperm morphology. Similarly, Chan et al [11]. found that seminal levels of PRL did not differ between normozoospermic and teratozoospermic men. On the contrary, Wijeratna et al. [34] reported that there was a significant negative relationship between seminal PRL levels and sperm morphology. In vitro incubation of human spermatozoa in the presence of PRL increases motility and decreases DNA damage in a dose-dependent manner [13] and stimulates cell metabolism [10]. Ufearo and Orisakwe [35] stated that an increase in endogenous PRL levels or administration of exogenous PRL improves sperm concentration and morphology in hypoprolactinemic infertile men treated with metoclopramide. It has been claimed that PRL increases the efficiency of spermatogenesis in seminiferous tubules by directly affecting steroidogenesis in Leydig cells [8] and improves the viability of sperm cells by activating Akt phosphorylation and reducing caspase activity [13].
Moreover, our findings showed that the abundance of HSP90 transcripts in ejaculated spermatozoa was significantly higher in infertile patients than in fertile individuals and was significantly related to sperm motility. Reduced sperm progressive motility was found to be significantly associated with higher sperm HSP90 transcript levels. Higher sperm HSP90 protein levels have been reported in oligozoospermic patients than in normozoospermic men [36]. However, Sagare-Patil et al. [37] found that HSP90 protein levels were lower in oligozoospermic patients than in normozoospermic controls and had a positive correlation with sperm count and motility. Lower levels of HSP90 protein were observed in the spermatozoa of oligoasthenozoospermic patients than in controls [37]. Tian et al. [38] reported that seminal HSPA2 transcripts differed significantly between normozoospermic men and asthenozoospermic patients and were negatively associated with sperm concentration and motility. Chan et al. [39] demonstrated that sperm HSP70 and HSP90 protein levels were elevated in infertile men with varicocele. Zhang et al. [22] found that HSP90 transcript abundance decreased in cryopreserved bovine sperm and was associated with sperm plasma membrane and acrosome integrity. Elevated HSP90 mRNA expression has been observed in the ejaculated sperm of oligozoospermic men [36]. Erata et al. [40] reported that HSP70 transcript abundance was significantly higher in asthenozoospermic and oligoasthenozoospermic infertile patients than in fertile controls.
HSPs play a major role in the process of spermatogenesis and sperm function [41]. Molecular disorders in the reproductive process can lead to abnormal sperm function. HSP90 interacts with cell division cycle 37 (CDC37) to regulate total protein threonine phosphorylation and SRC phosphorylation during human sperm capacitation [42]. Sun et al. [43] showed that HSP90 regulates human sperm capacitation via the Erk1/2 and p38 mitogen-activated protein kinase (MAPK) signaling pathways. Calle-Guisado et al. [44] reported that HSP90 protects the motility of porcine sperm during prolonged exposure to heat stress. HSP90 protects sperm function after freezing, and the abundance of HSP90 transcripts correlates with sperm viability [45]. HSP90 localizes in the neck, midpiece, and tail regions of human sperm and might play a crucial role in regulating sperm motility [41,46]. It has been suggested that HSP90 improves motility in spermatozoa by providing sufficient energy via inhibiting adenosine triphosphate (ATP) degradation [45]. Increased expression of HSP90 during the process of spermatogenesis can be due to its protective role against heat stress. HSP90 could improve sperm quality by activating nitric oxide synthase, which protects sperm from oxidative damage caused by ROS [47].
Furthermore, the present study showed that there was a significant association between seminal levels of PRL and the abundance of HSP90 transcripts in ejaculated spermatozoa. Higher seminal PRL levels were associated with lower HSP90 transcript abundance in ejaculated spermatozoa. It has been proposed that PRL exerts antioxidant activity [14,16], which in turn can reduce the adverse effects of oxygen free radicals on sperm cells. It can be inferred that a decrease in seminal antioxidant capacity causes oxidative stress, which in turn increases HSP90 expression. Given the proposed antioxidant properties of PRL, it is thought that seminal PRL depletion can lead to oxidative stress and LPO, thereby reducing sperm viability and motility and damaging sperm DNA [48]. Furthermore, it has been reported that HSP90 is involved in PRL-induced apoptosis signaling during spermatogenesis. Inhibition of the PRL receptor, as a client protein of HSP90, promotes spermatogonial apoptosis [25]. However, this study is subject to several limitations. Further studies with a larger sample size and specific methods, as well as experimental studies on the role of seminal PRL, are required.
This study showed that lower seminal PRL levels may be associated with impaired quality of sperm parameters in men. Given the proposed antioxidant properties of PRL and the relationship between seminal PRL levels and sperm HSP90 transcript abundance, it can be assumed that an imbalance between the seminal antioxidant capacity and the amount of free radicals leads to oxidative stress and reduced sperm quality in infertile patients. Therefore, in addition to the importance of assessing HSP90 transcript content in ejaculated spermatozoa, the present study suggests that seminal PRL levels can be analyzed as a diagnostic marker to evaluate semen quality and predict male fertility.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Author contributions
Conceptualization: MD, HG, MH. Formal analysis: MD, HG, MH. Methodology: MD, HG, MH.
Project administration: FI. Writing-original draft: MD, FI. Writing-review &editing: MD, HG.