Identification of royal jelly as a potential new drug to protect the ovarian reserve and uterus against cyclophosphamide in rats
Article information
Abstract
Objective
The aim of this study was to investigate the effect of royal jelly (RJ), a powerful natural antioxidant, on cyclophosphamide-induced ovarian damage.
Methods
Thirty-two Wistar albino rats were divided into four groups. Oral treatment was administered to all rats for 16 days after a single intraperitoneal injection. The control group received intraperitoneal and oral saline; the RJ group received intraperitoneal saline and 100 mg/kg/day oral RJ; the cyclophosphamide group received intraperitoneal 100 mg/kg cyclophosphamide and oral saline; and the treatment group received intraperitoneal 100 mg/kg cyclophosphamide and 100 mg/kg/day oral RJ. The groups were compared in terms of ovarian reserve tests and histopathological changes in the ovary and uterus.
Results
All follicle counts were higher in the treatment group than in the cyclophosphamide group. The increase in the number of preantral follicles (p=0.001) and the decrease in the number of atretic follicles (p=0.004) were statistically significant. RJ treatment significantly improved follicular degeneration and cortical fibrosis in the ovary and epithelial and gland degeneration in the uterus due to cyclophosphamide toxicity.
Conclusion
According to these results, RJ reduces cyclophosphamide-related ovarian and endometrial damage in rats. For this reason, it should be further investigated to determine its effects on reproductive function.
Introduction
Premature ovarian failure (POF) is defined as a decrease in ovarian folliculogenesis and steroidogenesis before the age of 40. Its etiology is often unclear, and in most cases, the cause is idiopathic. However, chemotherapy for any reason in reproductive age may cause iatrogenic POF by inducing granulosa cell apoptosis and follicular atresia, such as in cyclophosphamide (CP) treatment [1]. Due to the recent increase in cancer cases and the successful use of chemotherapy regimens, the incidence of POF related to chemotherapy is increasing [2]. For this reason, preserving fertility is important for women who desire to have children in the future and are scheduled for chemotherapy. Currently, there is no proven treatment for this purpose other than the cryopreservation of ovarian tissue, ova, or embryos [3]. Therefore, new studies are needed to find options to prevent POF caused by chemotherapy or to restore ovarian function in these women [2].
Royal jelly (RJ) is a natural superfood secreted from the hypopharynx and mandibular glands of worker bees [4,5]. In a bee swarm, except for the first 3 days, only the queen bee is fed RJ during its lifetime [6,7]. Unlike the sterile worker bees with the same genetic makeup, the spawn of the queen bee depends on it being fed with RJ. In addition, when there is no queen bee in a colony, one of the worker bees consumes RJ and turns into a “false queen,” regaining its ovulatory functions [8]. Therefore, RJ appears to be very important for ovulatory function.
RJ has been shown to protect some tissues, such as kidney [9] and bone marrow [10], against the harmful effects of CP. For this purpose, we conducted this experimental study to investigate whether RJ, which has not been tried in iatrogenic POF before, protects the ovarian reserve and uterus against the harmful effects of CP.
Methods
This study was initiated after approval was obtained from the Adiyaman University Animal Experiments Local Ethics Committee (2019/040). A total of 32 healthy Wistar albino female rats (24 weeks, 250 to 300 g) were used. All animals were reared under ideal laboratory conditions (12-hour dark/light cycle, 23°C±1°C, free access to water and food, plastic and metal cages designed for rats, four rats per cage). All procedures related to rats were carried out in humanitarian conditions and in accordance with ethical rules.
1. Source of RJ
The RJ used in this study was obtained from bee colonies in Kayapinar (37.91337, 40.08412, Diyarbakir, Turkey). In accordance with the cold chain rule, RJ was processed in the Beyçer beekeeping factory (06 980, Kahramankazan, Ankara, Turkey). After analysis (10-hydroxy-2-decenoic acid, 4.05%, pH 3.80, water 71%) by an authorized official institution (Chemistry Laboratory, Karadeniz Technical University), it was given orally to the rats after being stored again in accordance with the cold chain rules.
2. Experimental design
The rats were randomized into four groups by a laboratory staff member who was blinded to the content of the study. Each group was made up of eight rats. In the beginning, a vaginal smear was applied to rats, and those in diestrus were chosen for the experiment. The groups were formed as follows: for the control group, one intraperitoneal saline injection, and after 2 days, oral saline was given for 16 days. For the RJ group, 2 days after intraperitoneal saline administration, 100 mg/kg/day RJ was given orally for 16 days. For the CP group, a single intraperitoneal dose of 100 mg/kg CP was administered, and after 2 days, oral saline was given for 16 days. For the treatment group, a single intraperitoneal dose of 100 mg/kg CP was administered, and after 2 days, 100 mg/kg/day RJ was given orally for 16 days. The 16-day drug administration regimen was given once a day to all rats as oral gavage at a total volume of 1.5 cc. Oral treatment was started 2 days after the intraperitoneal CP injection to determine chemotherapy-related deaths.
3. Tissue and blood sampling
After 16 days of oral treatment, all rats were given general anesthesia with intramuscular (IM) 2 mg/kg xylazine hydrochloride (Xylazinbio, 2%, Bioveta, Ivanovice na Hané, Czech Republic) and IM 40 mg/kg ketamine hydrochloride (Ketosol, 10%, Richter Pharma, Weis, Austria). When adequate anesthesia depth was achieved (toe pinch stimulus), the abdominal skin was shaved, and antisepsis was given with a 10% povidone-iodine solution. The uterus and adnexa were removed after opening the abdomen with a midline incision. These tissues were placed in 10% formaldehyde for histopathological examination. Later, all rats were sacrificed by drawing intracardiac blood to study the follicle-stimulating hormone (FSH) and anti-Müllerian hormone (AMH) levels.
4. Determination of serum levels of AMH and FSH
Blood samples were centrifuged at 11,000 × g for 10 minutes, and sera were collected at the Department of Biochemistry, Medical School of Adiyaman and stored at −20℃ until analysis. The serum AMH levels were measured by an enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions (AMH Gen-II ELISA REF A79765, and AMH Gen-II calibrators and controls REF A79766, Beckman Coulter, Brea, CA, USA). The plates were read at 450 nm and with background wavelength correction at 620 nm. The results were expressed as ng/mL. The serum FSH levels were measured by ELISA according to the manufacturer’s instructions (Rat Follicle-stimulating Hormone ELISA Kit, Cat-No: EA0015Ra, Lot-No: 202012017, Bioassay Technology Laboratory, Shanghai, China). The FSH levels were expressed as mIU/mL. A Biochrom EZ Read 400 microplate reader was used to read the plates.
5. Histological procedure
Histopathological examinations were performed in the Adiyaman University Faculty of Medicine Histology and Embryology Laboratory. After fixing the ovarian and uterine tissues in a 10% formaldehyde solution for 1 week, they were embedded in paraffin blocks by routine histological follow-up with an automatic tissue tracking device (Leica TP1020, Nussloch, Germany). Ten 5-μm-thick serial sections were taken from the paraffin blocks of ovarian and uterine tissues using a Thermo Shandon Finesse ME microtome device (Thermo Fisher Scientific, Cheshire, UK). The sections obtained were stained with hematoxylin-eosin (HE) and Masson triple stains for histopathological examinations. Evaluations were made using the Carl Zeiss Axiocam brand ERc5 model (Carl Zeiss Microscopy GmbH, Jena, Germany) digital camera with an attached microscope.
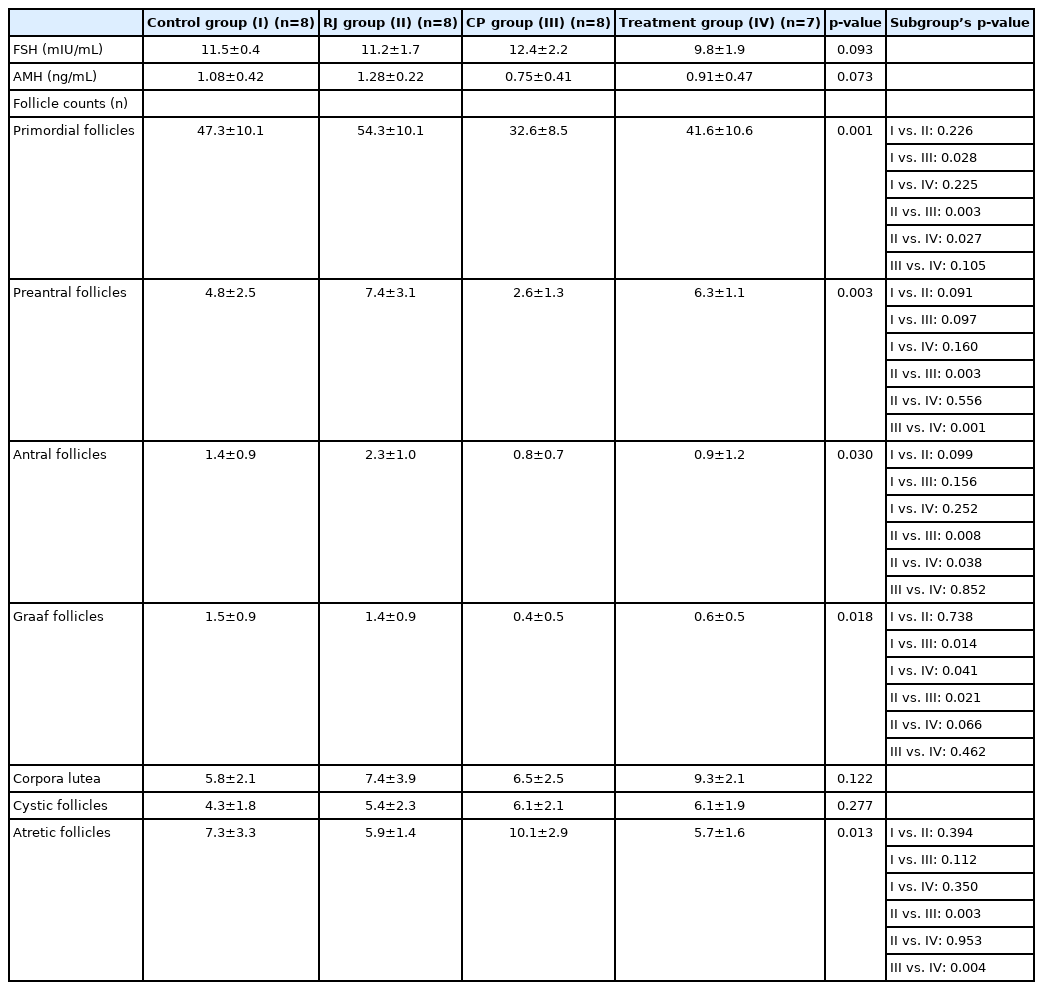
6. Follicle count
Serial sections of 10-μm thickness were taken from ovarian tissue with 1/30 sampling for the follicle count. The follicle count was carried out in 10 sections for each ovarian tissue sample. ImageJ software (National Institutes of Health, Bethesda, MD, USA) was used for counting (Figure 1). Follicle typing was performed as follows: the presence of oocytes surrounded by a single-layer of granulosa cells was considered a primordial follicle. The presence of oocytes surrounded by two or more layers of granulosa cells was considered a preantral follicle. The presence of oocytes with liquid between the granulosa cells was considered an antral follicle. The follicle with a largely fluid-filled antrum was considered a Graaf follicle. The ovulated ruptured Graff follicle was considered a corpus luteum. A follicle with impaired morphological integrity was considered an atretic (degenerative) follicle. A follicle in the form of a fluid-filled sac and cell mass was considered a cystic follicle.
Descriptive images of ovarian tissue prepared by hematoxylin and eosin (HE) and HE-Masson triple (MT) staining in the control group. (A) How to use the ImageJ method for follicle counting, (B) general view HE, ×4 magnification, (C) HE, ×10 magnification, (D, E) HE, ×40 magnification, and (F) MT, ×10 magnification. Green arrows, follicle with normal morphology; yellow arrows, cystic follicle; blue arrows, atretic follicle; red arrow, degenerate primordial follicle; black arrows, germinal epithelium. KL, corpus luteum; TA, tunica albuginea.
7. Histopathological evaluation
The histopathological evaluation of ovarian and uterine tissues was performed semi-quantitatively using histological scoring (H-score). If a lesion defined according to the H-score appeared to be less than 30% of the ×10 magnification area, it was considered less intense, and a score of 0 was given. If the lesion occupied 30% to 60% of the area in the image, it was considered moderate, and 1 point was given. If the lesion took up more than 60% of the area, it was considered very intense, and 2 points were given. Follicular degeneration (apoptosis and vacuolization in granulosa cells), stromal edema, vascular dilatation, and cortical fibrosis in ovarian tissue, as well as epithelial degeneration, endometrial gland degeneration, endometrial edema, and uterine fibrosis in the uterus, were evaluated using HE and Masson triple staining.
8. Statistical analysis
Data analysis was performed using the SPSS version 21 (IBM Corp., Armonk, NY, USA) package program. The AMH and FSH levels and follicle numbers were compared between groups using the Kruskal-Wallis test, and subgroup evaluations were conducted using the Mann-Whitney U test. Values are presented as the mean±standard deviation and median (range). Statistical significance was set to p<0.05.
Results
As one of the rats in the treatment group died on the 8th day of treatment, this group was completed with seven rats. The mean serum AMH and FSH levels and follicle numbers of the four groups are shown in Table 1. According to these results, the mean AMH and FSH levels were similar in all four groups.
When the four groups were compared in terms of follicle numbers, the mean number of primordial follicles was lower in the CP and treatment groups than in the RJ and control groups (32.6±8.5 vs. 41.6±10.6 vs. 54.3±10.1 vs. 47.3±10.1, p=0.001; respectively). The mean preantral follicle counts were higher in the treatment group than in the CP group (6.3±1.1 vs. 2.6±1.3, p=0.001). The mean antral follicle count was higher in the RJ group than in the CP and treatment groups (2.3±1.0 vs. 0.8±0.7 vs. 0.9±1.2, p=0.030; respectively). However, there was no statistically significant difference between the treatment group and the CP group.
The Graaf follicle counts were higher in the control and RJ groups than in the CP and treatment groups (1.5±0.9 vs. 1.4±0.9 vs. 0.4±0.5 vs. 0.6±0.5, p=0.018; respectively). However, there was no statistically significant difference between the treatment group and the CP group (p=0.462). The mean number of corpora lutea and cystic follicles was similar in all four groups. In addition, the mean number of atretic follicles was significantly lower in the treatment group than in the CP group (10.1±2.9 vs. 5.7±1.6, p=0.004).
In the histopathological evaluation (Table 2) of the ovarian tissue, a similar histological structure was found in the control and RJ groups (Figures 1 and 2). In both groups, cystic follicles and atretic follicles belonging to the previous cycle were observed in addition to follicles with a normal morphological appearance at different stages of development. The vascular structures had a normal appearance. In the general evaluation of the ovaries in these groups, no pathology was found except cystic and atresia follicles.
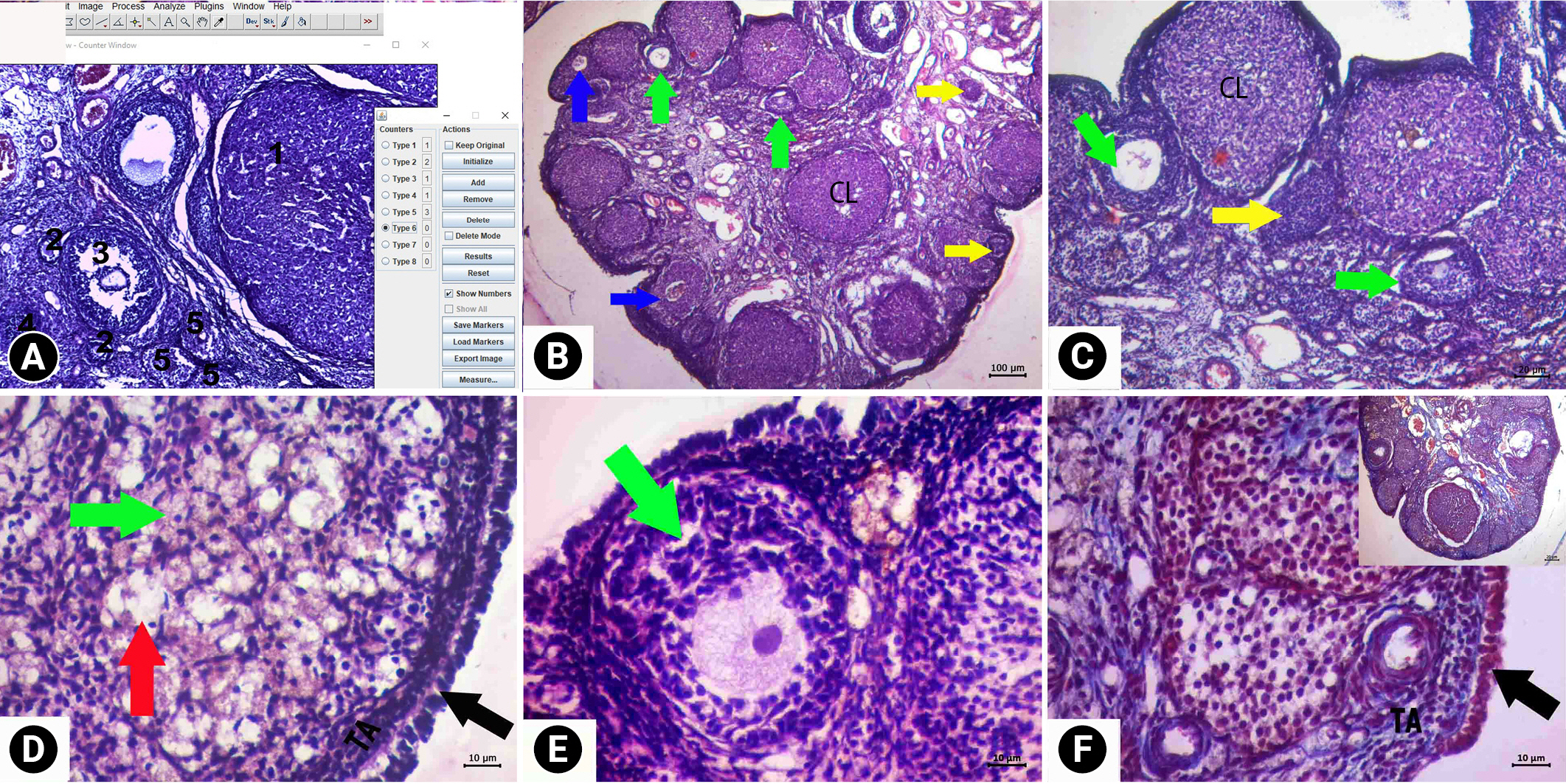
Descriptive images of ovarian tissue prepared by hematoxylin and eosin (HE) and HE-Masson triple (MT) staining in the royal jelly group. (A) General view HE, ×4 magnification, (B) HE, ×10 magnification, (C, D) HE, ×40 magnification, (E) MT, ×10 magnification, and (F) image of vacuolization in luteal cells and normal epithelial and connective tissue MT, ×10 magnification. Green arrows, follicle with normal morphology; yellow arrows, cystic follicle; blue arrows, atretic follicle; red arrows, degenerate primordial follicle; black arrows, germinal epithelium. KL, corpus luteum; TA, tunica albuginea.
When follicle density was examined in the CP group, the number of primordial follicles decreased significantly, and morphological deterioration was observed in the developing follicles (Figure 3). Intense findings of follicular degeneration were found in the examinations. Cortical fibrosis in the cortex and edema and vascular dilatation in the medulla were prominent. Corpus luteum degeneration was also apparent. Less follicular degeneration was detected in the ovaries of the rats in the treatment group than in the CP group (Figure 4). When the follicle density was examined, the number of primordial follicles was higher in the treatment group than in the CP group, and the rate of deterioration in their morphology decreased. Similarly, less morphological deterioration, stromal edema, and cortical fibrosis were found in the developing follicles.
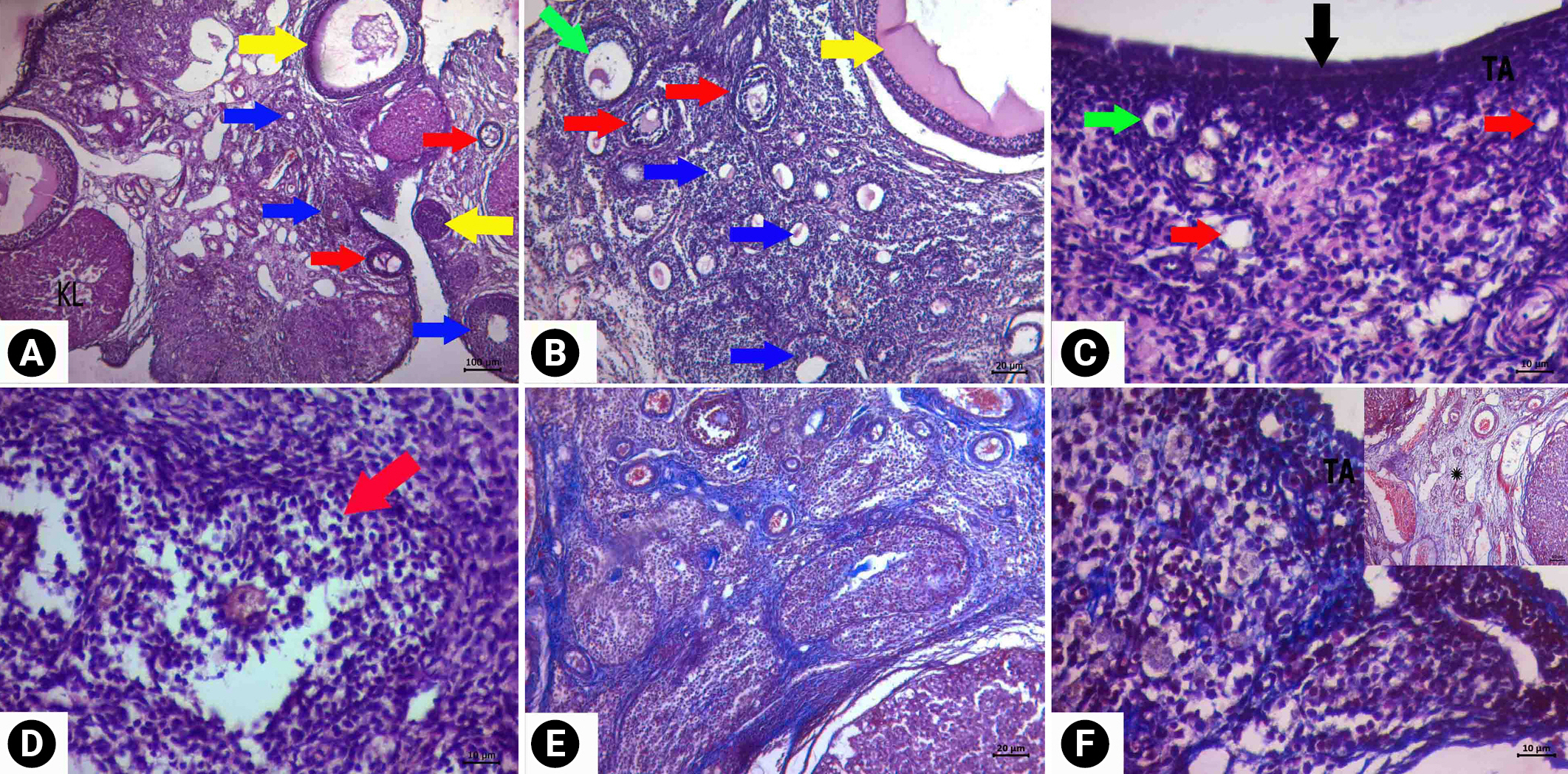
Descriptive images of ovarian tissue prepared by hematoxylin and eosin (HE) and HE-Masson triple (MT) staining in the cyclophosphamide group. (A) General view HE, ×4 magnification, (B) HE, ×10 magnification, (C, D) HE, ×40 magnification, (E) cortical fibrosis image MT, at ×10 magnification, and (F) image of vacuolization in luteal cells and normal epithelial and connective tissue MT, ×10 magnification (MT). Green arrows, follicle with normal morphology; yellow arrows, cystic follicle; blue arrows, atretic follicle; red arrows, degenerate primordial follicle; black arrow, germinal epithelium. KL, corpus luteum; TA, tunica albuginea.

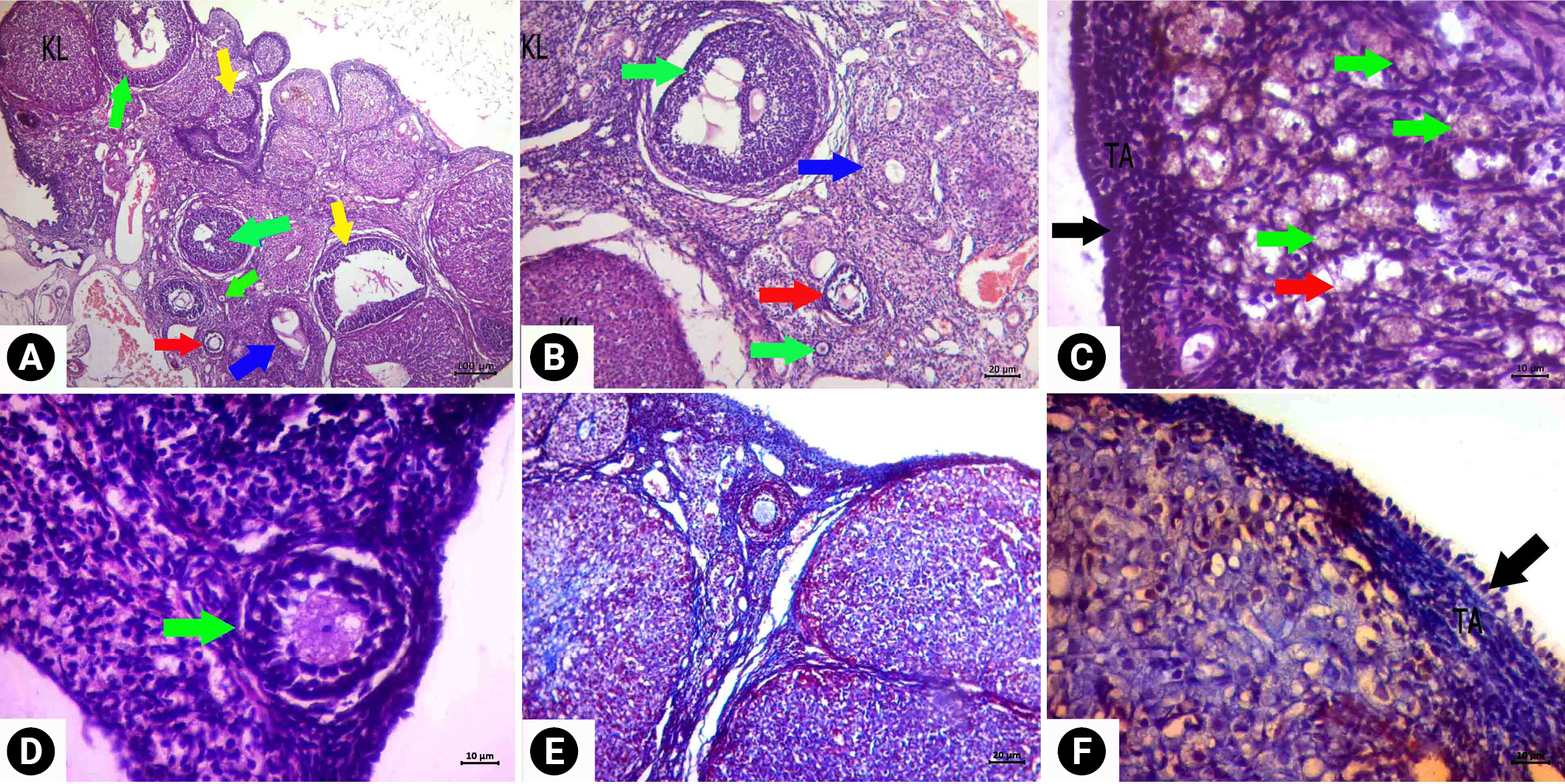
Descriptive images of ovarian tissue prepared by hematoxylin and eosin (HE) and HE-Masson triple (MT) staining in the cyclophosphamide and royal jelly group. (A) Image HE, ×4 magnification, (B) HE, ×10 magnification, (C, D) HE, ×40 magnification, (E) MT, ×40 magnification, and (F) image of reduced cortical fibrosis and edema compared to the cyclophosphamide group MT, ×10 magnification. Green arrows, follicle with normal morphology; yellow arrows, cystic follicle; blue arrow, atretic follicle; red arrows, degenerate primordial follicle; black arrows, germinal epithelium; asterisk, edema. KL, corpus luteum; TA, tunica albuginea.
In the histopathological evaluation of the uterine tissue, the control and RJ groups had similar histological findings (Figure 5). The endometrium, myometrium, and perimetrium layers had a normal appearance. The lumens of the glands were clearly visible, and the cells forming the gland epithelium formed a single-layered cubic epithelium. Centrally located oval nuclei of the single-layered prismatic epithelial cells lining the lumen were observed. There were collagen fibers showing a regular course in the connective tissues. No pathological findings were observed in the tissues of these groups.
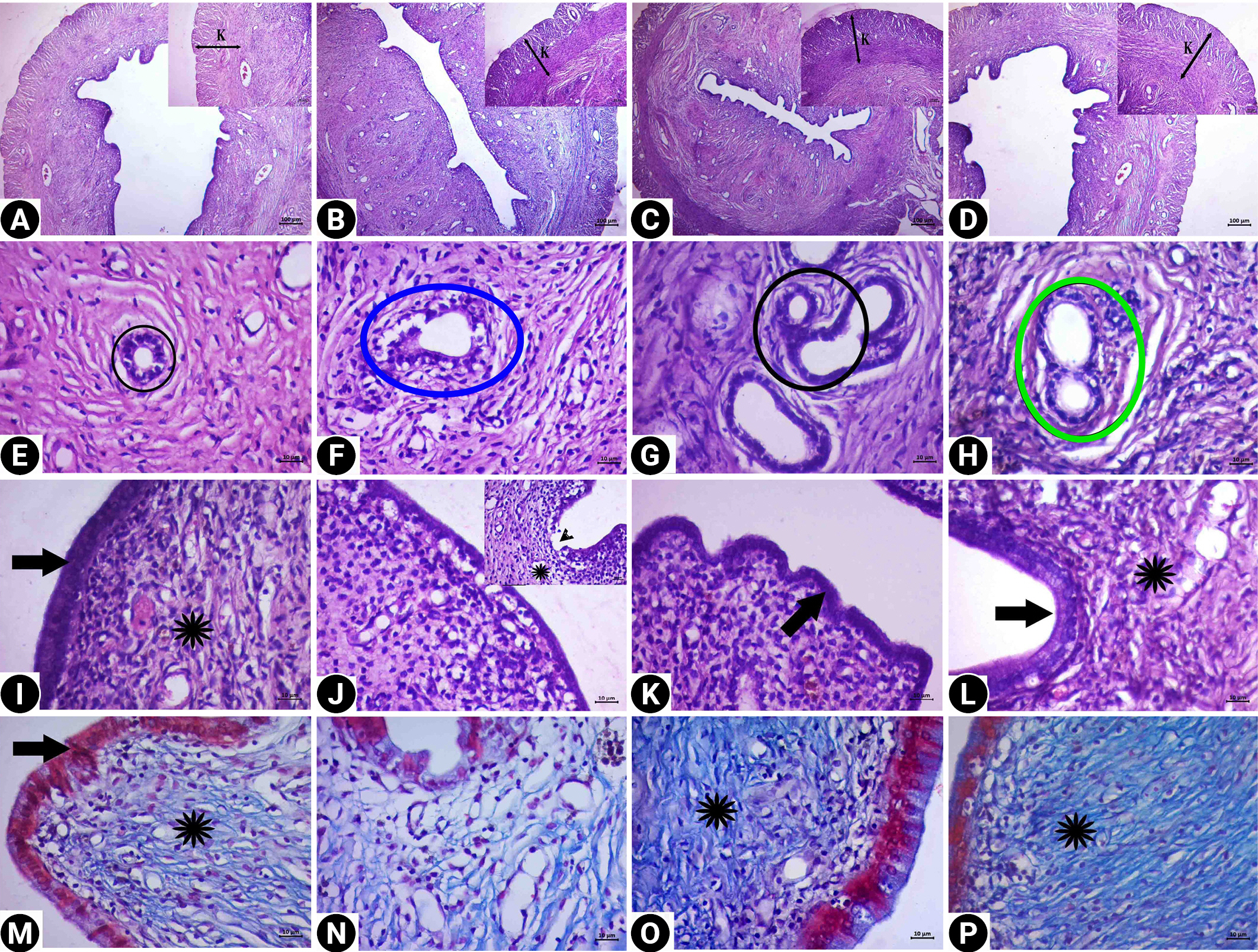
Descriptive images of uterine tissue prepared by hematoxylin and eosin (HE) and HE-Masson triple (MT) staining in the control group (A, E, I, M), cyclophosphamide group (B, F, J, N), royal jelly group (C, G, K, O), and the treatment group (D, H, L, P). (A, B, C, D) Image HE, ×4 magnification, (E, F, G, H, I, J, K, L) HE, ×40 magnification, and (M, N, O, P) MT, ×40 magnification. Black circles, gland (E, G); blue circle, degenerate gland (F); green circle, the integrity of the gland wall is preserved (H); asterisks, connective tissue; black arrows, epithelium (the morphology in the treatment group was similar to the control and royal jelly groups); arrowhead, degeneration of the epithelium (J). In the treatment group (D, H, L, P), epithelial vacuolization was reduced and the connective tissue area was similar to that in the control group. K, muscle layer.
Degeneration was detected in the cubic epithelial cells forming the epithelium of the glands in the CP group. In addition, the single-layer prismatic epithelium surrounding the lumen underwent changes and transformed into a flat and single-layer cubic epithelium, and signs of degeneration and vacuolization were observed in the epithelium. Edema and decreased collagen fibers in the connective tissue were observed. However, dilatation was detected in the stromal blood vessels. In the treatment group, the lumen of the glands was open, and the cells forming the gland epithelium preserved the single-layered cubic epithelial structure. A normal appearance was present in the single-layer prismatic epithelium surrounding the lumen. Edema was not observed in the connective tissue, and the morphological structure of the stromal blood vessels was found to be normal.
Discussion
This is the first study to show that RJ can protect the ovarian reserve against the harmful effects of CP. Ovarian reserve is the most important parameter that shows the reproductive function of a woman. The most important factors determining the ovarian reserve are the number of primordial follicles in the ovary and the consumption rate of primordial follicles [11].
CP, one of the most gonadotoxic chemotherapeutic agents that reduce the ovarian reserve, may exert this gonadotoxic effect by directly increasing apoptosis in primordial follicles [12,13] or by accelerating the transition to larger follicles [14,15]. The acceleration of the follicular transition makes the follicle pool more susceptible to the harmful effect of CP because of the higher mitotic activity. In recent studies, CP has been shown to damage the ovarian reserve with increased apoptosis in the granulosa cells at all stages of folliculogenesis [16,17]. In our study, consistent with the literature, all follicle numbers were found to be lower in the CP group than in other groups. In addition, the significant increase in the number of atretic follicles, follicular degeneration, and cortical fibrosis in the CP group indicates this gonadotoxic effect of CP.
CP decreases the ovarian reserve by increasing follicular atresia. It achieves this effect by changing the balance of anti-inflammatory and pro-inflammatory cytokines [18,19] and by damaging the granulosa cells through oxidative stress [20]. RJ, a superfood, prevents tissue damage due to oxidative stress by increasing antioxidant capacity and reducing oxidative stress [21,22]. This antioxidant effect, which protects cells against apoptosis [23], has been demonstrated in many tissues, such as the ovary, testis, kidney, and bone marrow [9,10,21,22]. RJ can protect the ovaries against CP’s oxidative stress-mediated cell damage because of this antioxidant effect that reduces apoptosis in granulosa cells and oocytes [24]. The statistically significant decrease in follicular atresia in the treatment group compared with the CP group in our study indicates the presence of this protective effect. Similarly, the detection of less ovarian follicular degeneration and cortical fibrosis and a higher number of preantral follicles in the treatment group supports this protective effect.
In the present study, the mean AMH level was found to be higher in the treatment group than in the CP group, although the difference was not statistically significant. The reason for the low AMH in the CP group may be that CP increases apoptosis in granulosa cells [16,17] and decreases the AMH synthesized from these granulosa cells [25]. A possible reason for the high level of AMH in the treatment group may be related to RJ’s protection of the follicular pool. Through the antiapoptotic effect of RJ, more granulosa cells survive with the acceleration of the transition to larger follicles in the follicle pool [14,15] through the effect of CP [21,22]. As a result, the increased AMH production suppresses follicular growth and causes a decrease in follicle consumption. The higher number of preantral follicles in the treatment group than in the CP group in our study supports this mechanism.
RJ consumption is the main reason for the longer lifespan and ovulation of queen bees, which have the same genetic makeup as infertile worker bees. Many experimental studies have shown this positive effect on fertility and pregnancy rates [26-28]. The protective effect of RJ on ovarian tissue observed in this study is consistent with this finding. However, it may not be sufficient to claim that RJ makes a positive contribution to reproduction only through folliculogenesis because an endometrium ready for implantation is required after fertilization. RJ can protect the endometrium, as well as other tissues [21,22], against the harmful effects of oxidative stress. In our study, the marked reduction of CP-induced glandular and epithelial degeneration in the RJ group suggests that RJ may also contribute to implantation.
One of the limitations of our study arises from its experimental nature. Due to the small number of animals, some differences between the groups were not found to be statistically significant. Another limitation is that different types and different doses of chemotherapeutic agents could not be compared with different doses of RJ. The fact that the effects of CP and RJ were not shown at the molecular level in our study can also be considered a limitation. However, the action mechanisms of both RJ and CP have been shown in many studies, making it unnecessary to recapitulate these results herein. The main purpose of our study was to investigate whether RJ can protect the ovarian reserve against chemotherapeutic agents. One of the most important advantages of this study is that it is the first one conducted for this purpose. This study also has the following methodological advantages. Many tests have been used to evaluate the ovarian reserve, but the most effective tests currently accepted are AMH levels and the antral follicle count [29]. Therefore, in this study, the effect of RJ on the ovarian reserve was evaluated using these two tests. In studies of folliculogenesis in the ovary, every rat in the experimental group should be in the same menstrual period. For this purpose, to eliminate any potential confusion in follicle count, we followed the rats with smears and started the experiment with rats in the diestrus phase.
In conclusion, according to the results of this study, the use of RJ protects the ovarian reserve against the gonadotoxic effects of CP. This protective effect may be related to a reduction in ovarian follicle consumption and degeneration and the elimination of cortical damage. RJ may also contribute to implantation by reducing epithelial and gland degeneration in the endometrium. In light of this information, we believe that RJ should be further investigated as a natural superfood that could improve the ovarian reserve and implantation in women suffering from infertility.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Author contributions
Conceptualization: MB, AT. Data curation: MB, EA, MO. Formal analysis: MB, OK. Funding acquisition: MB, AT, EA, OK, MO, DK, AMD. Methodology: MB, EA, OK, MO. Project administration: MB. Visualization: MB, EA, MO. Writing-original draft: MB, AT, AMD. Writing-review & editing: MB, OK, MO.
Acknowledgements
We would like to thank the veterinary surgeon Elif Tosun and the staff member Nurhan Tırascı for their assistance in the rats’ care, drug application, and surgery. We also acknowledge Dr. Mehmet Can Nacar for his contributions.