Evaluation of polyglycolic acid as an animal-free biomaterial for three-dimensional culture of human endometrial cells
Article information
Abstract
Objective
Animal-free scaffolds have emerged as a potential foundation for consistent, chemically defined, and low-cost materials. Because of its good potential for high biocompatibility with reproductive tissues and well-characterized scaffold design, we investigated whether polyglycolic acid (PGA) could be used as an animal-free scaffold instead of natural fibrin-agarose, which has been used successfully for three-dimensional human endometrial cell culture.
Methods
Isolated primary endometrial cells was cultured on fibrin-agarose and PGA polymers and evaluated various design parameters, such as scaffold porosity and mean fiber diameter. Cytotoxicity, scanning electron microscopy (SEM), and immunostaining experiments were conducted to examine cell activity on fabricated scaffolds.
Results
The MTT (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide) assay and SEM results showed that endometrial cells grew and proliferated on both scaffolds. Immunostaining showed cytokeratin and vimentin expression in seeded cells after 7 days of culture. On both scaffolds, an epithelial arrangement of cultured cells was found on the top layer and stromal arrangement matrix on the bottom layer of the scaffolds. Therefore, fibrin-agarose and PGA scaffolds successfully mimicked the human endometrium in a way suitable for in vitro analysis.
Conclusion
Both fibrin-agarose and PGA scaffolds could be used to simulate endometrial structures. However, because of environmental and ethical concerns and the low cost of synthetic polymers, we recommend using PGA as a synthetic polymer for scaffolding in research instead of natural biomaterials.
Introduction
The endometrium is a unique tissue lining the uterus that plays a critical role in the reproductive system by preparing a site for embryo implantation [1]. Advances in designing experimental models have resulted in a better understanding of the human endometrial environment and function. For decades, standard two-dimensional monocultures were typically used to study endometrial cell function; however, these traditional cultures fail to represent the complex three-dimensional (3D) architecture of the tissue [2]. Recent advancements in tissue engineering have led to the development of 3D tissue constructs utilizing matrices and a scaffold-based approach. A scaffold provides a mechanical framework that mimics the extracellular matrix (ECM) composition for cell growth [3-7]. Developing 3D cell culture models improves the ability to investigate the cellular and molecular features of tissue function and allows more accurate research on therapeutics [8]. The optimal 3D culture system conditions vary widely for each cell type or cell line [9]. Natural biomaterials are highly biocompatible and readily available, showing great potential for cell viability. Hydrogel-forming natural polymers have several advantages, including biomimicking of the ECM and self-assembling capability. They have excellent biocompatibility due to their high hydration rate and ability to engage in diffusion and exchange, facilitating cell function and viability [10-15].
One of the most commonly used hydrogel substrates in tissue engineering is fibrin, which has advantages such as a low price, good interactions between cells and the biomaterial, and a fibrillary and porous pattern. Fibrin is easy to handle [16,17], and fibrin hydrogels can be created from the patient’s plasma; thus, they can be considered as an autologous therapeutic product. Despite these advantages, the biomechanical properties of natural polymeric materials, such as fibrin hydrogels, are typically relatively poor compared to native tissues in terms of stiffness, flexibility, resistance, and strength. Hence, researchers have attempted to improve the biomechanical properties of fibrin hydrogels by combining them with another biomaterial. From this perspective, some studies have shown that the addition of agarose enhanced the biomechanical properties of fibrin hydrogels, mainly when chemical crosslinkers were used [18-22].
In tissue engineering, polymers derived from animal materials, such as fibrin and collagen, are used to create scaffolds structurally identical to those of the native ECM [23,24]. However, the dependence on animals has made these methods undesirable due to variability [25], environmental issues [26], and ethical concerns [27]. Non-animal or synthetic materials have emerged as a promising potential source for consistent, chemically defined, low-cost scaffolds. Synthetic or natural animal component-free polymers such as cellulose [28,29], chitin/chitosan [30], alginate [31], recombinant silk [32], polyglycolic acid (PGA) [1], polylactide [33], and polycaprolactone [34] provide low-cost, stable, and tunable scaffolds.
PGA is a common synthetic polymer that has been used to support diverse cell types, including fibroblasts and epithelial cells, to regenerate abdominal wall, urethral, and gut tissues [35-37]. The PGA polymer has good potential for high biocompatibility with reproductive tissues, and it is therefore a recommended suture material for perineal repair. It is also associated with a minimal tissue inflammatory response when used as a suture material for oral tissues compared to other sutures [38,39]. An electrospun PGA scaffold was selected to grow a 3D model of primary bovine endometrial epithelial and stromal cells that reflected the endometrium's architecture [1]. However, PGA scaffolds have not yet been studied for the 3D culture of human endometrial cells. Considering the advantages and disadvantages of natural and synthetic scaffolds, this study was designed to compare the functional reconstitution of the human endometrium using epithelial and stromal cells between synthetic polymer scaffolds and natural scaffolds.
Methods
1. Endometrial cell isolation
The Research Committee of Iran University of Medical Sciences reviewed and approved all aspects of this project with regard to ethical issues (IR.IUMS.REC 1396.32888). Written informed consent was obtained from all subjects. Endometrial biopsies (n=10) were obtained using a pipeline aspirator (Prodimed, Neuilly-en-Thelle, France) from fertile women during the proliferative phase of the uterus. Healthy fertile women (with regular menstrual cycles and at least one spontaneous conception) aged 20–35 years volunteered to participate in this study. Women who used intrauterine devices or received hormonal therapy during the previous 3 months were excluded. None of the participants had any gynecological pathologies.
All tissue samples were collected and labeled in Hank’s balanced salt solution (HBSS; Sigma, St. Louis, MO, USA) supplemented with 20% fetal bovine serum (FBS; Gibco, Waltham, MA, USA) and 1% penicillin/streptomycin (Sigma). The samples were processed immediately following primary isolation and washed by Dulbecco’s phosphate-buffered saline (DPBS; Sigma). Next, the samples were minced into 1×1 mm pieces using a scalpel in a Dulbecco's modified Eagle medium/F-12 nutrient mixture (DMEM-F12; Sigma). The dissected tissue was incubated for 1 hour at 37°C in a 10 mL digest solution, containing trypsin/EDTA (2.5 BAEE units/mL, Sigma), collagenase I (2 mg/mL, Sigma), and DNAse I (0.1 mg/mL, Sigma) in HBSS. After incubation, the digested tissue was strained in DMEM-F12 supplemented with 20% FBS (Gibco) and 1% penicillin/streptomycin (Sigma) through a 40 μm nylon mesh cell strainer. The stromal cells were passed through the filter and collected. The epithelial glands that were kept in the strainer were back-washed and collected. The stromal and epithelial glands were cultured into 75 cm2 and 25 cm2 culture flasks, respectively. All cell cultures were incubated at 37°C in a humid atmosphere with 5% CO2. Once the cell populations were ~70% confluent, they were frozen and stored in liquid nitrogen until further usage.
2. Three-dimensional endometrial cell co-culture
1) Three-dimensional cultures using fibrin-agarose scaffolds
Approximately 1.0 ×106 stromal cells were suspended in 32.7 μL of culture media, and 400 μL of human plasma was added to the mixture. In all steps of the 3D endometrial cell co-culture, 1 nM estrogen and 0.902 nM progesterone were added to the DMEM-F12 medium without phenol red, which was supplemented with 10% FBS (Gibco) and 1% penicillin/streptomycin (Sigma) and used as the culture medium. To prevent fibrinolysis, 3.8 μL of tranexamic acid (Amchafibrin; Rottapharm, Monza, Italy) was added. After that, 25 μL of melted type VII agarose (2% in DPBS) was added to achieve a final concentration of 0.1%. Finally, 38.5 μL of 100 mM CaCl2 was added to start fibrin polymerization. The mixture (total volume of 500 μL) was instantly transferred to a 24-well cell culture plate and incubated at 37°C to solidify. After 20 minutes, 500 mL of culture medium was added. Twenty-four hours after solidification, 3.0 ×105 epithelial cells were seeded on top of the scaffold. This was a modified version of Alaminos' 3D rabbit cornea culture protocol [40]. Figure 1A presents a schematic diagram of the 3D cultures performed using the fibrin-agarose scaffold.
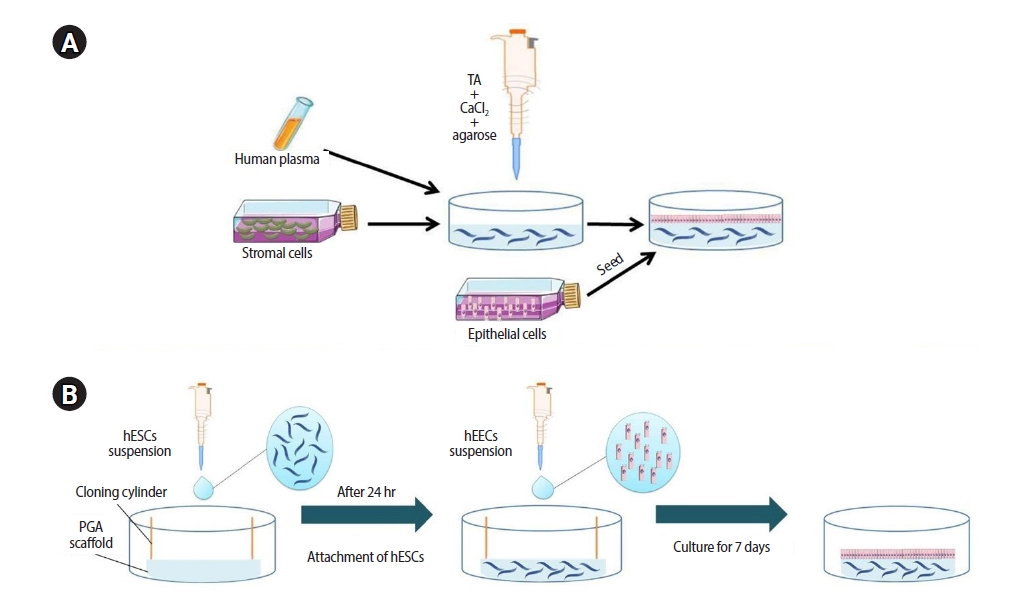
Diagrammatic scheme of the development of the three-dimensional (3D) endometrial culture systems. (A) A 3D matrix consists of stromal cells, human plasma, tranexamic acid (TA), CaCl2, and agarose. After 24 hours, epithelial cells are seeded on the top of the 3D matrix to form a monolayer. (B) Human endometrial stromal cells (hESCs) cultured on the polyglycolic acid (PGA) scaffold. After 24 hours, human endometrial epithelial cells (hEECs) are seeded on the top of the scaffold.
2) Three-dimensional cultures using PGA scaffolds
The first step was to fabricate PGA scaffolds by the electrospinning process. A 11.5% w/v solution of PGA (Lakeshore Biomaterials; Birmingham, AL, USA) in 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP; (Fluka, Milwaukee, WI, USA) was prepared, and complete overnight dissolution was performed using an orbital shaker. The solution of PGA in HFIP was dispensed through four blunt 21-gauge steel needles connected to a 10 mL plastic syringe with two syringe pumps at a flow rate of 0.04 mL/min. A 20-cm-long, 5-cm-thick steel mandrel covered in a sheet of non-stick release paper was placed 15 cm from the needle tip and rotated at 50 rpm. An 11.0 kV electrical field was applied between the paper-covered mandrel and the needle tip to perform electrospinning. Fibers were collected on the paper-covered mandrel and formed a nonwoven scaffold sheet. The electrospinning process was performed at 19°C and around 38% humidity. When deposition ended, the scaffold was removed and dehydrated under vacuum at 25°C for at least 72 hours. Next, 13-mm discs were cut from these scaffolds and kept in moisture-barrier pouches including a desiccant. The pouches were sterilized using gamma irradiation.
For 3D endometrial cell co-culturing, the PGA scaffold (13-mm diameter) was fixed to the well of a culture plate by an 8-mm cloning cylinder. To pre-wet the scaffolds, we filled the cloning cylinder with 300 μL of DMEM-F12. Approximately 1.0 ×106 stromal cells per scaffold in 200 μL of culture medium were seeded onto the wetted scaffold. After 4 hours, another 3,000 μL and 100 μL of DMEM-F12 was added to the outer and inner parts of the cloning cylinder, respectively. The stromal cell-seeded PGA scaffolds were incubated at 37°C in a humid atmosphere with 5% CO2. After 24 hours, 3.0 ×105 epithelial cells were seeded upon the scaffold. The culture medium was changed every 48 hours. Scaffolds were cultured for up to 7 days before being removed from the culture medium for histological examinations on days 1, 2, 5, and 7. Figure 1B presents a schematic diagram of the 3D cultures using the PGA scaffold.
3. Cytotoxicity assay
The MTT (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide) assay was applied to evaluate changes in the proliferation of viable cells seeded on the scaffolds. In the 24-well culture plates, the scaffolds consisted of epithelial and stromal cells that were free of culture medium and washed by DPBS. Next, 200 μL of MTT solution (5 mg/mL) was mixed with 800 μL of culture medium. The mixture was added to the scaffold and incubated at 37°C in a humid atmosphere with 5% CO2 for 3 hours. The MTT solution was removed. For cell lysis and the dissolution of formazan crystals, 200 μL of dimethyl sulfoxide (DMSO) was added. Then, 100 μL of DMSO–formazan solution was transferred to each well of a 96-well plate (SPL, Pocheon, Korea) and its optical density (OD) was measured at 570 nm absorbance with a plate reader (Polarstar Omega; BMG Labware, Aylesbury, UK). The MTT results for the scaffolds are reported as OD values.
4. Cell attachment assay
For the cell attachment assay, the culture medium was removed, and the scaffold structures were washed twice in DPBS for 5 minutes for fixation. The scaffolds were immersed in 2% paraformaldehyde for 5 minutes before being washed three times in DPBS. The scaffold structures were stored at 4°C in glutaraldehyde (2%) or paraformaldehyde (2%), respectively, for further processing (scanning electron microscopy [SEM] imaging and wax embedding).
1) Immunohistochemistry
The embedded scaffolds were evaluated with dual immunocytochemistry. Epithelial cells were identified with rabbit anti-cytokeratin (Abcam, Cambridge, UK) and stromal cells with mouse anti-vimentin (Sigma). Goat anti-rabbit immunoglobulin G conjugated with FITC (Abcam) and rabbit anti-mouse polyclonal antibody conjugated with Texas red (Jackson Immunoresearch, West Grove, PA, USA) used as secondary antibodies were diluted to 1:800 in phosphate-buffered saline (PBS) containing 1% bovine serum albumin (BSA). The scaffold sections were de-waxed in xylene two times, for 2 minutes each. Then, a graded series of ethanol (100%, 90%, 70%, and 50%) and deionized water were used for rehydration (2 minutes each). The slides were incubated in boiling sodium citrate buffer (pH 6.0) for 3 minutes, and then cooled and washed in PBS with 0.025% Triton X-100 (Sigma). Next, they were blocked in blocking solution (5% donkey serum+PBS+1% BSA) for 2 hours. Diluted primary antibody (1:100 in PBS containing 1% BSA) was added to the slides and incubated overnight at 4°C. Later, the slides were washed three times in PBS containing 1% BSA for 5 minutes each. Diluted secondary antibody was applied to the slides and incubated at room temperature for 1.5 hours in darkness. The slides were subjected to three washes in PBS containing 1% BSA and mounted using 4′,6-diamidino-2-phenylindole as the last step.
2) Scanning electron microscopy
For SEM, the scaffold structures were rinsed three times in DPBS and fixed in 2% glutaraldehyde for 3 days at 4°C. Then after washing with deionized water, a graded series of ethanol (30%, 50%, 70%, 95%, 100%, and 100% dry ethanol) was used to dehydrate the scaffolds. After dehydration, the specimens were frozen in an ultra-low temperature freezer (–80°C) and moved to a freezer dryer (Edwards Super Modulyo) for drying. Then, specimens were mounted and sputter-coated with gold. Samples were observed under SEM (AIS2300; Seron Technology, Uiwang, Korea) that was run at 20.0 kV.
5. Statistics
Data were presented as the mean±SEM and were analyzed using GraphPad Prism v9 (GraphPad, La Jolla, CA, USA). Data for the physical characteristics of the scaffolds and proliferation on scaffolds were compared using analysis of variance. A p-value <0.05 was considered to indicate statistical significance.
Results
1. Isolation and culture of endometrial epithelial and stromal cells
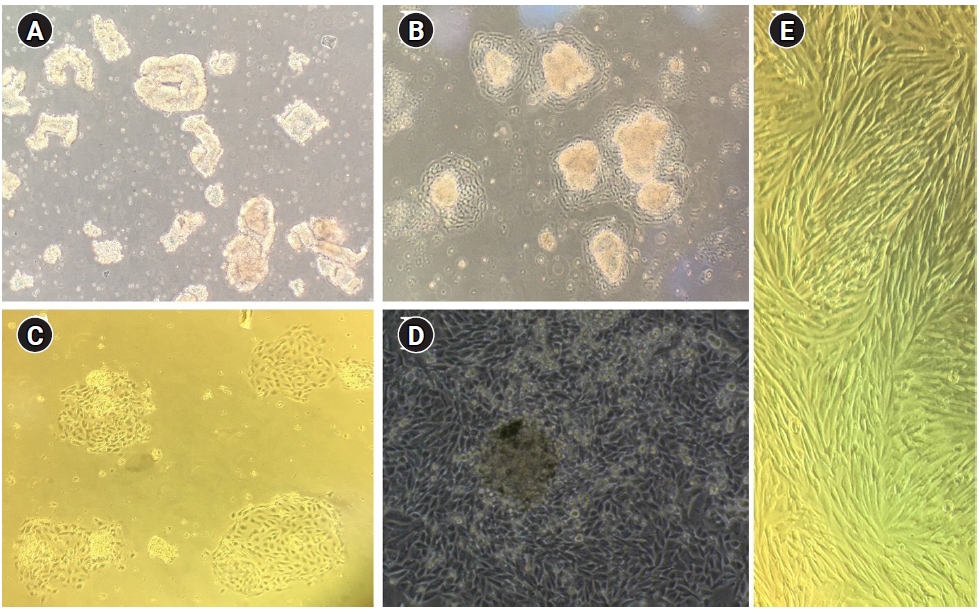
Human endometrial tissues obtained by a pipelle aspirator were enzymatically digested and expanded in two-dimensional cultures to achieve a sufficient number of cells. As described, the retaining epithelial glands in the strainer were back-washed and cultured in 25 cm2 culture flasks (Figure 2A). Epithelial glands attached to the flask within 24 hours (Figure 2B). Epithelial glands attached to each other extended and formed clusters that resembled islands (Figure 2C). These clusters eventually formed a monolayer of epithelial cells. In 5–7 days, a monolayer of confluent epithelial cells was achieved. At this stage, the epithelial cell monolayer folded over and formed dome-shaped structures (Figure 2D). The collected stromal cells from the filtrate were also cultured in a 75 cm2 culture flask. After 12 hours, the culture medium was changed to remove tissue debris, blood cells, and unattached epithelial cells. In 3–4 days, the stromal cells were confluent (Figure 2E). The medium in the cell culture was changed every 48 hours.
2. Initial assessment of the scaffolds
Electrospun PGA fibers were assembled into a dense mesh-like layer, as visualized by SEM (Figure 3). The physical features of both PGA and fibrin-agarose scaffolds were compared (Figure 4). The mean diameter of the fibers and porosity properties were not significantly different (the mean diameter of the fibers was 10.43 μm and 10.24 μm in the PGA and fibrin agarose scaffolds, the mean pore diameter of the PGA and fibrin-agarose scaffolds was 52.06 μm and 49.2 μm, and the largest pore size diameter in the PGA and fibrin-agarose scaffolds was 101.35 μm and 98.05 μm, respectively). Cell proliferation on scaffolds seeded with stromal and epithelial cells was assessed using the MTT assay. The number of growing cells in the co-culture could not be calculated from the OD values because the co-culture did not contain a unique cell type, and the MTT OD standard curve against cell number is specific for the cell type (Figure 5) [41].
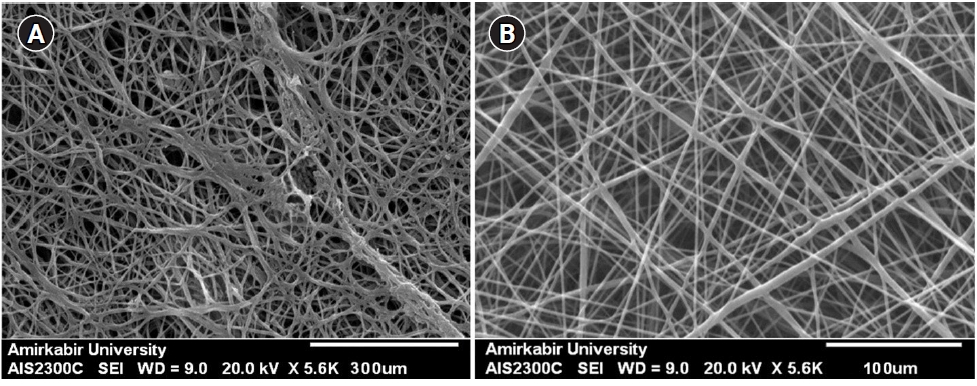
Scanning electron microscopy images of scaffolds prior to cell seeding. (A) Fibrin-agarose scaffold. (B) Electrospun polyglycolic acid scaffold.
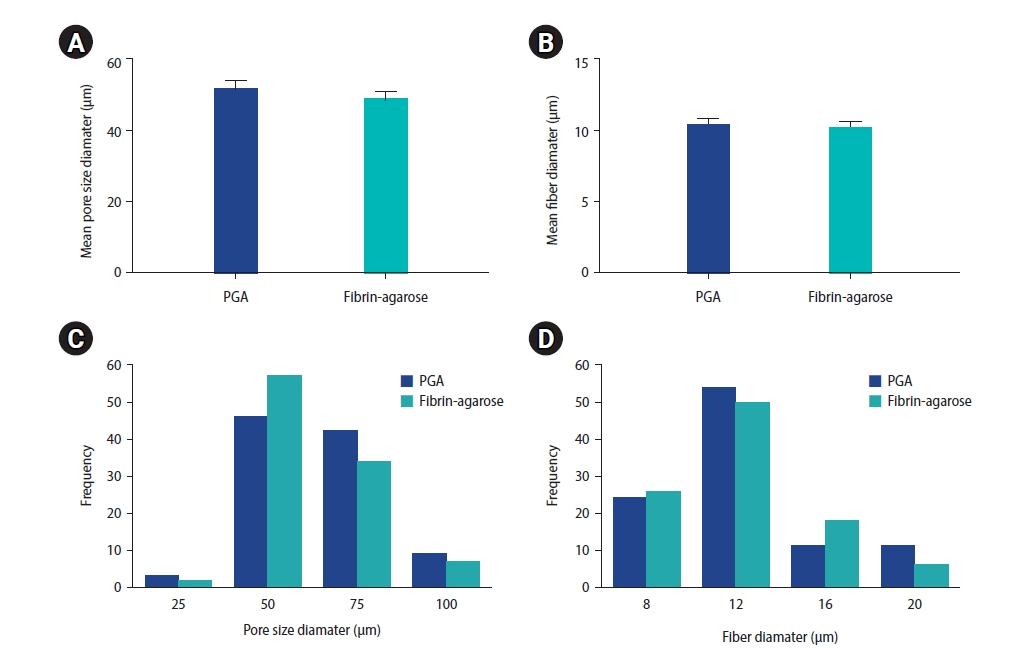
Comparison of physical characteristics between the fibrin-agarose scaffold and the electrospun polyglycolic acid (PGA) scaffold. Comparison of the mean pore size diameter (A) and the mean fiber diameter (B) in two scaffolds; these parameters were not significantly different (p≥0.05). Histogram of pore size diameter (C) and fiber diameter (D).
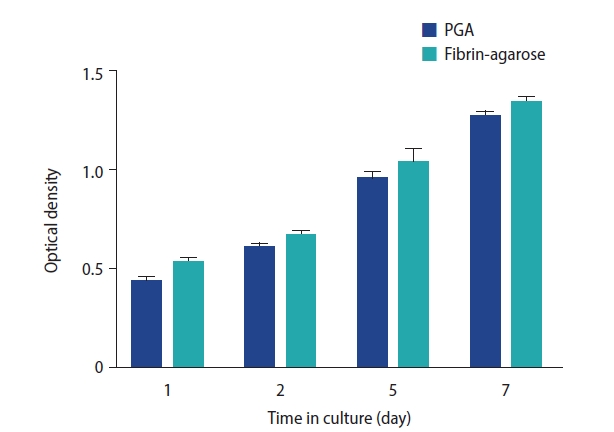
The MTT (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide) assay was used to measure the cell viability of stromal and epithelial cells co-cultured with the electrospun polyglycolic acid (PGA) scaffold or the fibrin-agarose scaffold. The MTT optical density of both the PGA and fibrin-agarose scaffolds seeded with stromal and epithelial cells increased during 7 days of cell culture. The cell viability did not show any significant between-group differences over time (p≥0.05).
The MTT OD of the fibrin-agarose scaffold seeded with stromal and epithelial cells increased during 7 days of cell culture (p<0.05). The MTT OD of the electrospun PGA scaffold seeded with stromal and epithelial cells also increased during 7 days of cell culture (p<0.05). The MTT OD of the fibrin-agarose scaffold was higher than that of the electrospun PGA scaffold at the same times of culture, but the difference was not statistically significant (p≥0.05) (Figure 5).
3. Description of the 3D co-culture of primary human endometrial epithelial and stromal cells
In the 3D culture, the origins and location of the epithelial and stromal cells were defined by cytokeratin and vimentin immunostaining, respectively. Immunohistochemistry (IHC) for cytokeratin was positive only for epithelial cells in the surface epithelium (Figure 6). IHC for vimentin was positive for the stromal cells located in the 3D matrix (Figure 6). On the top part of both 3D culture systems, epithelial cells formed a constricted cell monolayer. The stromal cells combined with the fibrin-agarose gel or PGA scaffolds became lengthened and expanded, displaying that the 3D culture systems supplied a suitable environment for the growth of endometrial cells.

Immunohistochemistry images of co-cultured epithelial and stromal cells seeded on fibrin-agarose and electrospun polyglycolic acid (PGA) scaffolds on day 7 of culture. Cross-sectional expression of vimentin (red) or cytokeratin (green) by endometrial stromal and epithelial cells on both scaffolds, respectively. DAPI, 4′,6-diamidino-2-phenylindole.
These results showed that both scaffolds—fibrin-agarose and PGA—could simulate the structure of the human endometrium. SEM was used to assess epithelial cell growth and proliferation on the scaffolds’ surfaces. The epithelial cell clusters were more distinct on the fibrin-agarose scaffold than on the PGA scaffold, which may indicate epithelial gland formation in this structure (Figure 7).
Discussion
In this study, fibrin-agarose (a natural biomaterial) and electrospun PGA (a synthetic, animal-free polymer) were selected as scaffolds to support the proliferation and growth of human endometrial constructs. The results of the MTT assay showed that neither of these two scaffolds had a toxic effect on the survival of endometrial cells. IHC showed that stromal cells grew inside the scaffold and epithelial cells formed a monolayer on the matrix. These cells were able to grow on the scaffolds, and these scaffolds were able to create an endometrial-like structure, as IHC proved.
MacKintosh et al. [1] designed a new 3D culture system for bovine endometrial cells using electrospun PGA as a scaffold, and Wang et al. [42] used a fibrin-agarose scaffold to simulate the human endometrial structure. As shown by the results for cell adhesion and proliferation on the scaffolds, both scaffolds were suitable for primary human endometrial epithelial and stromal cells. The scaffolds maintained the growth of a monolayer of epithelial cells upon multiple layers of stromal cells, similar to natural endometrial tissue architecture. The PGA electrospun scaffold has a significant advantage, in that it synthetically mimics the ECM protein, collagen, and provides a perfect context to support the growth of tissue [43,44]. However, PGA nonwoven scaffolds have several limitations, such as a high degradation rate and poor mechanical properties. PGA electrospun scaffolds have been broadly used in the cell culture of different tissues, including human skin and vascular tissue and bovine endometrial and aortic endothelial cells [45,46]. However, to the best of our knowledge, this report is the first model of human endometrial cells growing on PGA electrospun scaffolds.
A scaffold is expected to support the attachment and growth of cells, provoke ECM deposition, and have proper porosity to facilitate gas diffusion, molecular signaling, and waste and nutrient products to enable the differentiation and survival of cells [47]. As demonstrated using IHC, both scaffolds simulated the human endometrial structure. In both culture systems, epithelial cells formed a single layer of tight cells on the top of the scaffolds. Stromal cells were embedded into the fibrin-agarose gel, or PGA scaffolds became extended and spread out, proving that these systems of 3D culture provided a proper environment for cell growth.
In an endometrial 3D model, Wang et al. [42] detected spontaneous gland formation by epithelial cells in the 3D constructs of stromal cells, probably from epithelial cell contamination within the population of stromal cells or from differentiation of uterine stem cells into epithelial cells during culture. We did not recognize epithelial glands by IHC on either scaffold. However, SEM imaging showed that clustered forms of epithelial cells grew upon the fibrin-agarose scaffolds, representing endometrial glands. The proliferation of stromal cells co-cultured with epithelial cells was observed over time on both PGA electrospun and fibrin-agarose scaffolds. The capability of these scaffolds to facilitate the growth of both stromal and epithelial cells was proven using histology and the MTT assay. We focused on embedding stromal cells inside the scaffold, on top of which a suspension of epithelial cells would be seeded. The growth of stromal cells within the scaffolds and growth of the epithelial cells on the superficial layer of the scaffold was confirmed using immunocytochemistry. On the electrospun scaffold, most stromal cells were concentrated on the upper part of the scaffold and developed into and on top of the scaffold, which led to an increase in the thickness of the entire structure. The growth and expansion of stromal cells within the scaffolds showed that the scaffold's porosity was adequate for cell transfer, and stromal cells were also detected in the deeper parts of the scaffolds.
Our results showed that both scaffolds facilitated the growth and proliferation of endometrial cells and could create endometrial-like structures. Therefore, due to the environmental and ethical concerns about animal resources in relation to natural polymers such as fibrin and the reasonable price of synthetic scaffolds, we recommend using synthetic scaffolds such as PGA to create endometrial-like structures in research studies. While the endometrial arrangement reported in this study only included epithelial and stromal cells, normal endometrium also contains glands, a vascular system, and immune cell populations. Although establishing an endometrial culture system using the two major cell types (epithelial and stromal cells) is beneficial, the involvement of other related cell types in the co-culture model could be investigated in further studies using methods such as organoids.
This study presents a straightforward model in which, similar to a fibrin-agarose (a natural biomaterial) scaffold, multiple layers of human endometrial stromal cells were cultured in 3D on a PGA electrospun scaffold (a synthetic, animal-free polymer) overlaid by a monolayer of human endometrial epithelial cells, and the overall arrangement was similar to the native endometrium. Replacing animal-derived hydrogels using a synthetic scaffold, such as electrospun PGA, has many potential benefits in clinical applications in terms of physiological, and structural properties, as well as environmental and ethical considerations. The accessibility of an advanced 3D model of endometrial tissue will also be useful for studying diseases and developing treatment methods. However, further studies are needed to assess and improve these models.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Author contributions
Conceptualization: FSA, MA. Data curation: SA, FSA. Formal analysis: ZB, MM, PBM, RA. Methodology: SA, AAS, LG. Project administration: MA. Visualization: SA, FSA. Writing–original draft: SA. Writing–review & editing: FSA, AAS, ZB.
Acknowledgements
The authors would like to express their special gratitude to members of the Anatomy Department, School of Medicine, Iran University of Medical Sciences. Moreover, the authors also would like to acknowledge all staff at Shahid Akbar Abadi Hospitals, without whose cooperation this process could not have been completed.