Platelet-rich plasma treatment in patients with refractory thin endometrium and recurrent implantation failure: A comprehensive review
Article information
Abstract
Refractory thin endometrium and recurrent implantation failure are among the most challenging infertility-related factors hindering successful pregnancy. Several adjuvant therapies have been investigated to increase endometrial thickness and the pregnancy rate, but the treatment effect is still minimal, and for many patients, these treatment methods can be quite costly and difficult to approach. Platelet-rich plasma (PRP) is an autologous concentration of platelets in plasma and has recently been elucidated as a better treatment option for these patients. PRP is rich in cytokines and growth factors, which are suggested to exert a regenerative effect at the level of the injured tissue. Another advantage of PRP is that it is easily obtained from the patient’s own blood. We aimed to review the recent findings of PRP therapy used for patients with refractory thin endometrium and recurrent implantation failure.
Introduction
Refractory thin endometrium and recurrent implantation failure (RIF) are the most challenging infertility factors hindering successful pregnancy. An endometrial thickness of more than 7 mm is crucial for endometrial receptivity, and a thin endometrium is related to poor pregnancy outcomes and an increased risk of recurrent pregnancy loss [1]. Several adjuvant therapies have been attempted to increase the endometrial thickness, using medications such as estradiol hormonal supplementation [2], vasoactive agents such as low-dose aspirin [3], vaginal sildenafil [4], intrauterine infusions of granulocyte colony-stimulating factor [5], and stem cell treatment [6,7]. However, the treatment effect on improving the endometrial thickness and pregnancy rate is still minimal, and for many patients, these treatment methods can be quite costly and difficult to approach.
RIF is defined as a status when a woman under the age 40 years fails to become pregnant after transfer of at least four good-quality embryos in a minimum of three fresh embryo transfer or frozen embryo transfer (FET) cycles [8]. The pathophysiology of RIF is multifactorial, but uterine and embryonic factors are generally considered to be the most important causes [9]. In order to increase endometrial receptivity, previous treatments such as hysteroscopy to remove intracavitary fibroids, polyps, adhesions, or septa showed some improvements in increasing the likelihood of pregnancy. However, when none of these etiologies is noted and the endometrial thickness is also normal, clinicians have limited further options that they could try to increase the endometrial receptivity.
Platelet-rich plasma (PRP) is an autologous concentration of platelets in plasma that has recently been elucidated as a better treatment option for these patients. PRP is rich in cytokines and growth factors (GFs), which have been suggested to exert some regenerative effects at the level of the injured tissue [10]. Furthermore, due to the important roles of cytokines and GFs in the embryonic implantation process [11], PRP has been a “rising star” in improving endometrial receptivity. Another advantage of PRP is that it is easily obtained directly from the patient’s own blood. We aimed to review the recent findings of PRP therapy used for patients with refractory thin endometrium and RIF.
Methods
A literature search was done using PubMed to investigate recently reported PRP studies in the reproductive endocrinology field. The search period was from January 2018 to May 2022 to find the most up-to-date studies regarding PRP use in endometrium-related infertility. The search words included “platelet-rich plasma,” “gynecology,” “infertility,” and “endometrium.” Mostly case reports, pilot studies with small sample sizes, and a few randomized controlled trials (RCTs) were found.
1. PRP preparation
There is still no consensus on a standardized protocol for preparing therapeutically effective PRP. The basis of PRP preparation is mainly the differential centrifugation of the whole blood [12]. Each component of the whole blood is separated into different layers by centrifugation due to differences in specific gravity. Two main methods are known for preparing PRP: the PRP method and the buffy-coat method [12]. In the PRP method, fresh blood is obtained by venipuncture in acid citrate dextrose tubes and centrifuged right away using soft spin. The supernatant plasma containing platelets is separated and centrifuged at a higher speed (hard spin) to obtain a platelet concentrate. The lower third is PRP, and at the bottom platelet pellets are formed. The buffy-coat method uses whole blood stored at 20°C–24°C and centrifuged at a “high” speed. Due to its density, three layers are formed: red blood cells at the bottom, platelets and white blood cells in the middle, and platelet-poor plasma (PPP) on top. The PPP layer is removed, and the buffy-coat layer is transferred to another tube for centrifugation at low speed to separate white blood cells. Alternatively, a leukocyte filter can be used.
Arora and Agnihotri [13] described the importance of anticoagulants in preparing PRP. Anticoagulant citrate dextrose-A is the most commonly used anticoagulant in commercial kits since it maintains an optimal pH for platelets at 7.2. The citrate binds to calcium and prevents the coagulation cascade. They also emphasized the importance of minimizing the PRP’s surface area in contact with the atmosphere (using small diameter tubes with caps) in order to stop CO2 from diffusing into the plasma and increasing the pH. An increased pH may potentially cause spontaneous aggregation of the platelets, making it difficult to utilize PRP.
Several commercial PRP preparation kits are available internationally, but there is substantial heterogeneity in the concentrations of platelets, leukocytes, and GFs in PRP. No general consensus exists regarding the optimal component concentrations [14]. Future research should focus on finding the most suitable PRP concentration for applications in infertility.
2. Proposed PRP mechanism
The mechanism through which PRP acts on refractory thin endometrium has not yet been definitely established, but it is believed that several GFs play important roles. Platelets are anucleated cytoplasmic fragments of megakaryocytes that contain α-granules with various GFs [15,16]. GFs are known to control angiogenesis, cell proliferation, stem cell migration, and inflammation [17]. For refractory thin endometrium, angiogenesis and cell proliferation may be the key mechanisms that need to be enhanced to stimulate the recovery process. Among the GFs in α-granules, platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), transforming growth factor-β (TGF-β), fibroblast growth factor, and insulin-like growth factor are considered to be important in the effects of PRP [18]. PDGF has several effects on the endometrium; it exerts a mitogenic effect in endometrial stromal, decidual, and epithelial cells; enhances DNA synthesis in endometrial stromal cells; stimulates the chemotactic migration of endometrial stromal cells; and promotes endometrial stromal cell motility [19]. VEGF stimulates neovascularization through its endothelial chemokine and mitogenic properties [20]. TGF-β has been shown to regulate endometrial decidualization, the uterine immune response, and endometrium repair during menstruation [21]. Fibroblast growth factor initiates angiogenic processes in the endometrium, upregulates VEGF receptor 2, and promotes endothelial proliferation and organization [22]. Insulin-like growth factor induces endometrial proliferation through the protein kinase B (AKT)/mammalian target of rapamycin (mTOR) pathway and initiates endometrial cell decidualization [23] (Table 1).
Interestingly, the α-granules contained in PRP have both pro- and anti-angiogenic properties. In order for PRP to promote angiogenesis, it is necessary to activate the pro-angiogenic cell surface receptors (VEGF, PDGF, TGF-β1, epidermal GF, serotonin, angiopoietin-1 and -2, matrix metalloproteinase-1 and -2, and interleukin-8) [24]. Some studies have shown that higher concentrations of PRP attenuated the endometrial cell proliferation rate and led to negative results [25,26]. This is speculated to be due to an excess amount of GFs resulting in the activation of an increased amount of anti-angiogenic factors (TGF-β1, plasminogen activator inhibitor, thrombospondin, angiostatin, endostatin, platelet factor 4, CXCL4L, tissue inhibitors of metalloproteases) to hinder cell proliferation. Giusti et al. reported in an in vitro study that 1.5×106 platelet/µL was the optimal concentration of activated platelets for promoting angiogenesis of human endothelial cells, but further in vivo studies are required to implement this recommendation in a clinical setting [26].
3. PRP use in refractory thin endometrium and Asherman’s syndrome
Molina et al. [27] prospectively evaluated 19 patients with a history of refractory thin endometrium to whom PRP was given by intrauterine injections. In all cases, endometrial thickness reached >9 mm after the second PRP injection. The pregnancy rate was 73.7%, of which 26.3% yielded live births and 26.3% ongoing pregnancies.
Chang et al. [28] investigated a larger study population of 64 patients with refractory thin endometrium (<7 mm) and administered intrauterine injections of PRP to 34 patients. The PRP group had significantly thicker endometrium than that of the control group. The implantation and clinical pregnancy rates in the PRP group were significantly higher than in the control group (27.94% vs. 11.67%, p <0.05; 44.12% vs. 20%, p <0.05, respectively).
Kim et al. [29] studied 22 patients with a history of two or more failed in vitro fertilization (IVF) cycles and refractory thin endometrium of <7 mm. Their prospective interventional study compared the study participants’ previous non-treated and later PRP-treated FET cycles. PRP was injected two or three times from menstrual cycle day 10 of the FET cycle, and FET was done 3 days after the final PRP injection. The implantation, clinical pregnancy, and live birth rates were 12.7%, 30%, and 20%, respectively in the PRP-treated cycles, while all previous cycles reported rates of 0%. However, the endometrial thickness showed no significant difference between the PRP and previous non-treated cycles.
A preliminary study using a mouse model of Asherman’s syndrome (AS) was done by Kim et al. [30] to assess the effectiveness of human PRP for endometrial recovery. Three separate experiments were performed. First, the effects of PRP on endometrial regeneration were assessed by evaluating the endometrial histology and expression of fibrosis-related factors. Second, the mice implantation sites and embryo weights were compared between the PRP and control groups. Third, live births were compared. Human PRP improved endometrial morphology, reduced the degree of fibrosis, and downregulated the expression of fibrosis markers. Higher numbers of implantation sites and live births were also noted.
A consecutive study by the same group of authors used a mouse model of AS to discover the molecular mechanisms of PRP that act on damaged endometrium [31]. They showed that the GFs in PRP promoted angiogenesis by increasing proangiogenic factors such as Hif1α, Hif2α, VEGF-α, Ang-1, Hgf, and Igf-1. PRP also promoted the migration of endometrial stromal cells to injured uterine areas, leading to uterine regeneration in pathologic conditions. Furthermore, PRP significantly increased the phosphorylation of STAT3, which is a critical transcription factor for tissue remodeling and regeneration, in both stroma and epithelial compartments in uteri with AS. Additionally, the mice that received PRP treatment had significantly higher mean weights of embryos and their placentas than the control mice, suggesting that PRP treatment considerably alleviates intrauterine growth restriction phenotypes in AS.
de Miguel-Gomez et al. [32] conducted an in vitro composition analysis and murine model of AS to study the effect of PRP from different sources on endometrial damage. The authors [32] tested whether plasma from human umbilical cord blood had stronger effects than adult PRP (aPRP) on endometrial recovery. The in vitro cell proliferation and migration rate after treatment with umbilical cord plasma was the highest, and aPRP also revealed a significant increment. The mouse model study showed higher expression of Ki67 and Hoxa-10 in the endometrium after applying aPRP, and the proteomic analysis revealed a specific protein expression profile. The damaged uterine tissue showed more pro-regenerative markers after the application of umbilical cord plasma than after other treatments (nonactivated umbilical cord plasma, activated aPRP, and no treatment).
An interesting novel PRP injection method was reported by Agarwal et al. [33] by injecting PRP in the endo-myometrial junction hysteroscopically. Thirty-two patients with a refractory thin endometrium received hysteroscopic PRP injections, and 24 of them (75%) had improved endometrial thickness (>7 mm). They underwent FET and among them, 10 had clinical pregnancies with positive fetal heartbeat and two had biochemical pregnancies.
The most recent prospective interventional study, reported in January 2022, found that PRP optimized endometrial thickness in both fresh and FET cycles [34]. Twenty women with refractory thin endometrium (<7 mm), regardless of hormone replacement therapy, underwent 26 PRP cycles during fresh embryo transfer and FET. PRP infusions were repeated every 48 hours if needed, and the maximum number of PRP infusions was limited to 3. The mean endometrial thickness increased significantly after PRP infusion (p<0.001) with average increases of 1.07 mm and 0.83 mm after the first PRP treatment (p<0.001) during fresh IVF and FET, respectively. The clinical pregnancy rates, implantation rates, and live birth rates were not significantly different between fresh embryo transfer and FET cycles (p>0.05) (Table 2).
4. PRP use in RIF
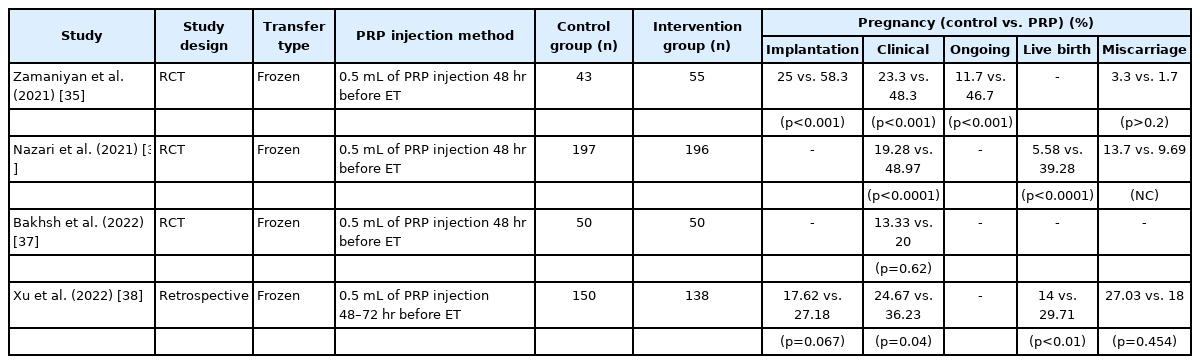
Zamaniyan et al. [35] investigated 98 RIF patients (who failed to become pregnant after three or more transfers of good-quality embryos) in a RCT. Fifty-five patients were given intrauterine PRP infusions 48 hours before embryo transfer in FET cycles. The other 43 patients comprised the control group, and these two groups showed significant differences in clinical (52.7% vs. 23.3%, p=0.003) and ongoing pregnancy rates (50.9% vs. 16.3%, p<0.001). Interestingly, although the study participants already had normal endometrial thickness, the PRP-treated group showed significantly increased endometrial thickness compared to the control group (13.15±1.42 mm vs. 10.00±0.93 mm, p<0.001).
The largest RCT was reported in 2021 by Nazari et al. [36], including 418 women with a history of RIF (failure to achieve pregnancy after three or more embryo transfers with high-quality embryos) undergoing FET. Patients were randomly assigned to PRP and control groups. The PRP group received 0.5 mL of PRP by intrauterine injection 48 hours before FET. Among the 418 candidates, 393 participants completed the study (PRP: n=196; control: n=197) and higher chemical pregnancy, clinical pregnancy, and live birth rates were observed in the PRP group (p<0.0001, p<0.0001, p<0.0001, respectively). There were no significant differences in the rates of multiple pregnancies and pregnancy complications. Only the spontaneous abortion rate was lower in the PRP group than in the control group.
The most recent RCT study, published in January 2022 by Bakhsh et al. [37], found that 100 women with an unexplained RIF history (previously failed to conceive after three or more transfers of high-quality embryos) had positive pregnancy outcomes after using PRP . These patients, undergoing FET, were divided randomly into PRP and control groups. The pregnancy rate was 20% in the PRP group and 13.33% in the control group, but this difference did not reach statistical significance (p=0.62). The authors still concluded that PRP may play a role in improving the fertility status of RIF patients and that larger RCT studies are needed.
Xu et al. [38] retrospectively evaluated 288 women with a RIF history (three or more consecutive failed embryo implantations with good-quality embryos, defined as at least six cleavage-stage embryos or three blastocysts). In total, 138 patients with PRP treatment and 150 patients who did not receive treatment were compared and the implantation, clinical pregnancy, and live birth rates were higher in the PRP group. Except for the implantation and miscarriage rates, the other results were statistically significant (Table 3).
Conclusions
Autologous PRP injections have shown substantial benefits as a feasible method to treat refractory thin endometrium and RIF. Recent studies are gathering evidence in support of the hypothesis that the GFs in PRP increase endometrial receptivity. However, larger-scale, high-quality RCTs will be needed to address some of the issues and determine the proper PRP preparation and dosage necessary to effectively treat endometrium-related infertility.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Author contributions
Conceptualization: HS, SWL, WSL. Data curation: MKK. Formal analysis: MKK. Methodology: MKK, SWL. Project administration: WSL. Visualization: HS, SWL. Writing–original draft: MKK. Writing–review & editing: MKK, SWL.