Cytokine supplementation of in vitro human embryo culture media: A randomized controlled trial of effects and cost-effectiveness
Article information
Abstract
Objective
This study aimed to evaluate the impact of supplementing a standard single-step culture medium (SSCM) with three cytokines (CYK) and different concentrations of human serum albumin (HSA) on the ongoing pregnancy rate (OPR) and cost-effectiveness ratio (CER) after intracytoplasmic sperm injection (ICSI) cycles.
Methods
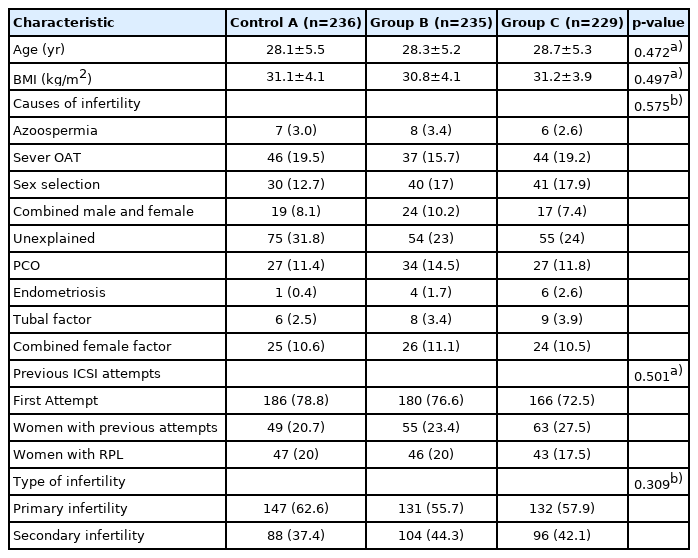
Patients were randomly allocated at the time of oocyte culture to one of three groups (A, B, or C): group A (control; n=236), culture in SSCM plus 5 mg/mL HSA, without CYK supplementation; group B (n=235), culture in SSCM supplemented by CYK plus 5 mg/mL HSA; and arm C (n=229), culture in SSCM supplemented by CYK plus 2.5 mg/mL HSA. This randomized controlled trial (trial registration no: NCT04547699) included 700 women for whom ICSI was planned between September 2019 and August 2021. The primary outcome was OPR, and the CER per ongoing pregnancy was included as a secondary outcome.
Results
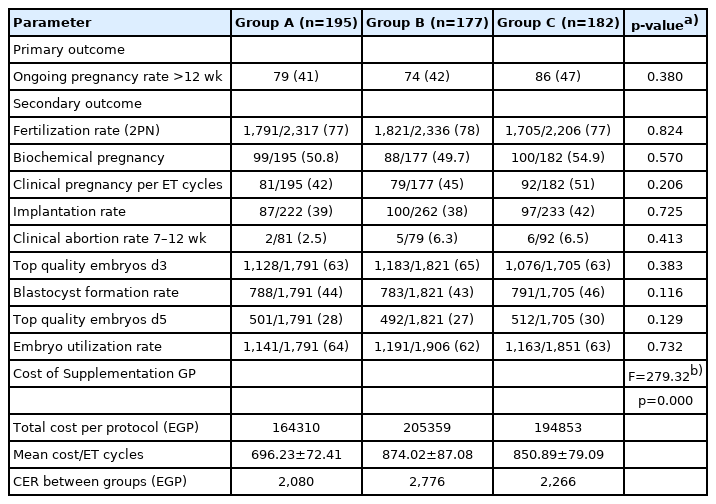
No statistically significant difference was found among groups A, B, and C in the OPR, which was 79/195 (40.5%), 74/177 (41.8%), 86/182 (47.3%) per embryo transfer cycle in each group, respectively (p=0.380). Statistically significant differences were observed in the CER, which was 2,080 L.E per ongoing pregnancy in group A compared to the CYK-supplemented groups (2,776 and 2,266 L.E per ongoing pregnancy in groups B and C, respectively) (F=279.32, p=0.000).
Conclusion
SSCM without CYK supplementation was the most cost-effective culture protocol. Further studies are needed to evaluate the effectiveness of integrating CYK into in vitro human embryo culture media before using it in routine clinical practice.
Introduction
The in vitro fertilization (IVF) embryo culture medium is a critical component in the laboratory process of handling gametes and embryos in vitro, which affects preimplantation embryo development, the likelihood of ongoing pregnancy or early pregnancy loss, and the birth weight of newborns [1-5]. The in vivo microenvironment is dynamic and includes a large number of cytokines and growth factors required for preimplantation embryo development, whereas the IVF culture medium does not exactly mimic the in vivo environment [5-7]. Commercial culture media containing nutrients, vitamins, and growth factors are now widely available. However, no evidence shows that adding additional costs by supplementation of IVF culture media is cost-effective for improving the ongoing pregnancy rate (OPR) in infertile couples [8].
The integration of cytokines into human embryo culture media has shown associations with various embryonic and clinical outcomes after intracytoplasmic sperm injection (ICSI). Thus, the long-term effect of cytokine enrichment of media is still unclear and warrants further studies with longitudinal follow-up to evaluate its effectiveness [9]. The costs of IVF treatment are considered as the first burden for infertile couples due to rising healthcare costs and the fact that infertility care is not a covered benefit in many countries [10]. Artificial reproductive technology treatments are fully or partially funded by the government in most high-income countries [11], while patients in low- or middle-income countries such as Egypt usually have to self-fund infertility treatments [12,13]. This fact has an impact on patients through both financial hardship and treatment discontinuation. Therefore, the balance between costs and effectiveness must be considered before introducing any add-ons into IVF general practice [14]. Cost-effectiveness analysis (CEA) is a method of assessing whether the current mixture of interventions is efficient, as well as whether a proposed new technology or intervention is appropriate; it is therefore suitable for situations where a valid and reliable estimation of the benefits of alternative options is not a meaningful possibility [15].
This randomized controlled trial (RCT) aimed to evaluate the impact of supplementing the standard single-step culture medium (SSCM) with a combination of three cytokines and different human serum albumin (HSA) concentrations on ICSI cycle outcomes and the cost-effectiveness of culture medium supplementation compared to non-supplemented cycles.
Methods
Out of 1095 recruited patients, 700 were enrolled in this RCT (Figure 1). Patients were randomly allocated at the time of oocyte culture into one of three arms (A, B, or C). In group A, the culture medium was prepared with the standard SSCM and 5 mg/mL HSA, without cytokine supplementation. Group B had the medium prepared with SSCM supplemented with cytokines and a high protein concentration (5 mg/mL HSA). The medium in group C was supplemented with cytokines and a low protein concentration (2.5 mg/mL HSA).

Flowchart of patient enrolment. SSCM, single step culture medium; ITT, intention-to-treat; CYK, cytokines; HSA, human serum albumin; ET, embryo transfer; PGD, pre-embryo transfer genetic diagnosis; OHSS, ovarian hyper stimulation syndrome; RPL, recurrent pregnancy loss.
1. Design and duration
This prospective, multi-center study was performed between September 2019 and August 2021 at Mansoura Integrated Fertility Center, Mansoura, Egypt, and Rahem Fertility Center, Zagazig, Egypt.
2. Eligibility and consent
All women provided written informed consent before the study was conducted. The trial received ethical approval from the Institutional Review Board of Zagazig University (No. ZU-IRB#9277), and ethical approval was obtained by each participating center from the local board of directors, since this was a multi-center RCT. This RCT (trial registration no:NCT04547699) was conducted in accordance with the Declaration of Helsinki [16] and the CONSORT (Consolidated Standards of Reporting Trials) [17] statement for reporting parallel groups. Patients were included based on the following inclusion criteria: patients aged 18–38 years who fit the medical definition of infertility (no pregnancy despite 1 year of regular unprotected intercourse). The exclusion criteria were a history of ovarian or adnexal surgery, suspicious findings of ovarian malignancy, the presence of endocrine disorders (e.g., diabetes mellitus, hyperprolactinemia, thyroid dysfunction, congenital adrenal hyperplasia, Cushing syndrome, and adrenal insufficiency), patients with poor ovarian response (defined as fewer than two mature oocytes retrieved at the day of OPU), and a history of male globozoospermia.
3. Randomization and blinding
After receiving informed written consent from participants, we randomized patients according to an opaque envelope indicating the treatment group. Information on patients’ allocation was randomly placed in opaque, sequentially numbered envelopes by a person, uninvolved in the trial. An embryologist selected the next envelope in the sequence on the day before oocyte retrieval to allow for overnight preparation of the culture medium.
The culture dishes were prepared with patient identifiers, and the media type was recorded by a data entry clerk. Clinicians, embryologists, and participants in the two centers had no access to information on the allocation of randomized patients. Embryologists who were not involved in oocyte and embryo handling supplemented the culture medium with cytokines and prepared the culture dishes according to random allocation numbers in the opaque envelopes, which corresponded to one of the three groups (A, B, or C).
4. Cytokine supplementation and culture
Group A (control) was prepared with the standard SSCM and in-house protein supplementation with the common concentration used in commercial culture media (5 mg/mL HSA; LGPS, LifeGlobal, Beernem, Belgium) without cytokine supplementation. Group B (cytokine plus a high HSA concentration of 5 mg/mL) was prepared with SSCM (LifeGlobal) supplemented with cytokines and a high HSA concentration (5 mg/mL HSA). Cytokines were diluted in culture medium directly, as they are water-soluble. In-house protein supplementation was performed with 5 mg/mL HSA (LGPS, LifeGlobal) with 2 ng/mL granulocyte macrophage colony-stimulating factor (GM-CSF; G5035, Sigma-Aldrich, St. Louis, MO, USA), 5 ng/mL heparin-binding epidermal growth factor (HB-EGF; E4643, Sigma-Aldrich) and 5 ng/mL leukemia inhibitory factor (LIF) (SRP9001, Sigma-Aldrich). The preparation of group C involved SSCM (LifeGlobal) and in-house protein supplementation with 2.5 mg/mL HSA (LifeGlobal) with 2 ng/mL GM-CSF (G5035, Sigma-Aldrich), 5 ng/mL HB-EGF (E4643, Sigma-Aldrich), and 5 ng/mL LIF (SRP9001, Sigma-Aldrich).
5. Oocyte retrieval, sperm preparation, ICSI, embryo transfer, and luteal phase support
Oocyte retrieval was performed 35 hours after triggering under ultrasound guidance. Semen analysis was performed according to the World Health Organization guidelines [18], and sperm preparation was conducted using the standard density gradient centrifugation method (45% and 90% PureSperm; Nidacon Ltd., Gothenburg, Sweden) diluted in SpermRinse(Vitrolife, Göteborg, Sweden). Mature oocytes were then inseminated by ICSI and cultured according to a randomized protocol in groups A, B, or C. Embryos were either freshly transferred after oocyte retrieval or freezing and thawing in consecutive frozen embryo transfer (FET) cycles. All embryos were cultured in a humidified incubator (Labotect C200-Box, Göttingen, Germany) at 37°C, under 6% CO2 and 5% O2. Each oocyte was incubated directly after OPU in an Oosafe center well dish with two compartments (Oosafe; Sparmed, Denmark) for 2 hours before denudation. Each 35-mm Oosafe dish held five 50-µL droplets for culturing denuded oocytes until ICSI (15±5 minutes), and each 60-mm Oosafe culture dish held 10 35-µL droplets for culturing oocytes (2–3 oocytes per droplet) following ICSI and until embryo transfer (day 0 to day 5). Dishes were prepared overnight from the cytokine or control medium overlaid with paraffin oil (LifeGlobal). We adjusted the culture conditions to 37°C±0.1°C and 6.0% CO2, 5% O2, and N2 balanced at a pH of 7.27±0.02 (as measured for each batch of medium by a blood gas analyzer). Embryo development was evaluated according to morphological criteria according to the 2011 Istanbul consensus workshop[19]. Luteal phase supplementation was applied differently according to the use of fresh embryo transfer or a different endometrium preparation method in FET cycles.
6. Culture media and cost calculations
The cost of each culture medium protocol was individually calculated by direct estimation in an Excel sheet, including the costs of SSCM, HSA, and cytokine supplementation by multiplying the volume of consumed media by the costs of the prescribed protocols for fresh embryo transfer cycles in the current study.
7. Primary and secondary outcomes
The primary outcome was the OPR at gestational week 12, as determined by ultrasonography. The secondary outcome measures were (1) the cost-effectiveness ratio (CER), calculated by dividing the different costs of culture media of the two protocols by the numerical difference in the OPR according to the equation below:
(2) the fertilization rate, defined as fertilized oocytes with two pronuclei (2PN) per number of metaphase II (MII) oocytes×100; (3) top-quality embryos on day 3, defined as embryos with eight blastomeres of stage-proper sizes, and <10% fragmentation by volume; (4) the blastocyst formation rate, defined as the number of differentiated blastocysts on day 5/6 per number of 2PN zygotes in the same cycle×100; (5) the biochemical pregnancy rate, defined as the number of women with a positive serum human chorionic gonadotropin test at ≥14 days after embryo transfer expressed per 100 embryo transfer cycles; (6) the clinical pregnancy rate, defined as the number of clinical pregnancies (diagnosed by ultrasonography) expressed per 100 embryo transfer cycles; (7) the implantation rate, defined as the number of gestational sacs determined by ultrasonography per number of embryos transferred×100; (8) the clinical pregnancy loss rate, defined as pregnancy loss at 7–12 weeks of gestational age divided by the number of clinical pregnancies×100; (9) the embryo utilization rate, defined as the number of embryos utilized (transferred or cryopreserved) divided by the number of 2PN zygotes in the same cycle×100. All outcome measures and embryo scoring were based on the International Glossary of Infertility and Fertility Care, 2017 [20]. Embryos were assessed according to the 2011 Istanbul consensus workshop [19].
8. Cost-effectiveness analysis
The main outcome was measured based on the number of ongoing pregnancies per culture protocol. The CER was calculated by dividing the total cost of the culture media within the protocol (numerator) by the number of ongoing pregnancies (denominator).
9. Statistical analysis
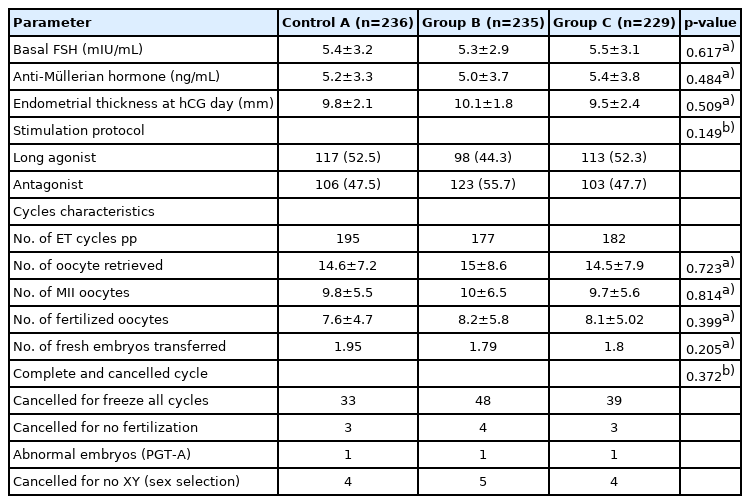
Data were statistically described in terms of mean±standard deviation, median and range, or frequencies (number of cases) and percentages when appropriate. Because the groups were large enough, numerical variables between the study groups were compared using one-way analysis of variance. For comparing categorical data, the chi-square test was performed. The exact test was used instead when the expected frequency was less than 5. Two-sided p-values less than 0.05 were considered statistically significant. To compare cost-effectiveness, a CEA analysis was chosen because the current literature and health technology assessment guidelines recommend doing so even when there is no significant difference in outcomes (as was the case for our RCT). Sensitivity analyses were carried out to evaluate the robustness of our model. All statistical calculations were done using IBM SPSS ver. 22 (IBM Corp., Armonk, NY, USA). The sample size (n=700 women, power of 80%) was based on the hypothesis of a 12% increase in the OPR at gestational week 12.
Results
The study groups were comparable regarding their baseline characteristics (age, body mass index, infertility diagnosis, previous attempts, and infertility types), as displayed in Table 1. The ovarian stimulation protocols, cycle characteristics, number of completed and canceled ICSI cycles, baseline levels of follicle-stimulating hormone and anti-Müllerian hormone, and endometrium thickness on the trigger day were comparable between the study groups (Table 2). Twenty-six percent of the participants were diagnosed with unexplained infertility, while 20% and 17% were diagnosed with male and female factor infertility, respectively. The mean number of transferred embryos was 1.95, 1.79, and 1.81 in groups A, B, and C, respectively (p=0.205).
1. Clinical outcomes
No statistically significant difference was observed among the study groups in terms of the OPR: 79/195 (40.5%) in group A (control), 74/177 (41.8%) in group B, and 86/182 (47.3%) in group C (2.5 mg/mL) (p=0.339 ). The biochemical pregnancy rate per embryo transfer cycle was 99/195 (50.8%) in group A, 88/177 (49.7%) in group B, and 100/182 (54.9%) in group C (p=0.574). The clinical pregnancy rates were 81/195 (41.5%), 79/177 (44.6%), 92/182 (50.5%) in groups A, B, and C, respectively (p=0.206). The clinical pregnancy loss (abortion) rates at 7–12 weeks of gestation were 18/99 (18%) in group A, 9/88 (10%) in group B, and 8/100 (8%) in group C (p=0.072). No statistically significant differences were observed in the fertilization, implantation, blastocyst formation, and embryo utilization rates (p=0.824, p=0.725, p=0.116, and p=0.732, respectively).
2. Subgroup analysis
Statistically significant differences were observed among a subgroup of 136 women (Table 4) with a previous history of recurrent pregnancy loss (RPL; a history of loss of two or more clinical pregnancies prior to 22 completed weeks of gestational abortion/miscarriage) [20]. The OPRs within the RPL subgroup were 12/47 (25.5%), 18/46 (39.1%), and 22/43 (51.1%) in groups A, B, and C, respectively (p=0.043), while the clinical pregnancy and pregnancy loss rates were comparable (Figure 2). Although the mean cycle costs in the subgroups were similar to those obtained in the overall study, with group A having significantly lower costs than the other groups, the CER of group C was the lowest. In the RPI subgroup, group C had a lower CER due to the significantly lower rate of pregnancy loss in this subgroup.
3. Costs of culture media supplementation
The costs were 696 L.E (95% confidence interval [CI], 687–706 L.E) in group A, 874 L.E (95% CI, 862– 884 L.E) in group B, and 851 L.E (95% CI, 841–862 L.E) in group C. The CER was 2,724 L.E per ongoing pregnancy for group A, 2,174 L.E per ongoing pregnancy for group B, and 1626 L.E per ongoing pregnancy for group C (Tables 3(-(5).
Discussion
Several studies have demonstrated that cytokine supplementation of IVF culture media had no effect on embryonic chromosomal constitution [4,21,22]. The concentration of each cytokine in our study was similar to those in prior RCTs [4,22] and half of the concentrations used in some other studies [25,26]; these concentrations were also tested for safety and stability in both murine and human studies [4,27,28]. The present study differed from other previous studies in that we performed CEA to evaluate the CER for the supplementation of cytokines in IVF culture media. We added low and high HSA concentrations to the cytokine-supplemented media (groups B and C), but not to the SSCM culture media (group A or control) with a low HSA concentration because SSCM clearly showed suboptimal performance outcomes in previous studies [4]; thus, we used SSCM supplemented with 5 mg/mL HSA because that concentration is commonly used in IVF culture media. The results of this study demonstrated that no statistically significant differences were found in clinical pregnancy, pregnancy loss (clinical abortion at 7–12 weeks) and ongoing pregnancy outcomes among the studied groups. Compared to the standard medium (SSCM), no significant differences were found in embryo quality, the blastocyst formation rate, embryo utilization rate, or the biochemical pregnancy rate in cytokine supplemented groups after fresh (ICSI-embryo transfer) cycles.
A significantly higher OPR was observed in a subgroup of 136 patients who had a previous history of RPL when the culture medium contained a low HSA concentration (2.5 mg/mL). Among this subgroup of RPL patients, each pair of groups was compared using the Fisher exact test after applying the Bonferroni adjustment for multiple comparisons revealed a significantly higher OPR in group C, in which culture was performed in medium supplemented with cytokines combined with low HSA (2.5 mg/mL), than in the control group (A) (p=0.048). In contrast, no significant differences were found between groups B and C (p=0.29) or between groups A and B (p=0.187). A significantly lower rate of pregnancy loss at 7–12 weeks of gestation was observed in group C than in group A (p=0.009). No significant differences in the rate of pregnancy loss were found when comparing groups B versus C (p=0.186) or A versus B (p=0.104). However, Fawzy et al. [] added recombinant HSA (rHSA) instead of HSA, although the use of rHSA is limited in IVF culture media due to its additional cost with no evidence of improved results [29]. Moreover, HSA is widely used for IVF culture medium supplementation combined with cytokines and growth factors [4,30,31].
Our results are consistent with some previous studies [32-37], but different from those of others [4,22,28].The impact of cytokine supplementation in vitro is still debated in animal and human research, although several studies indicated improvement in blastulation rates after the incorporation of GM-CSF in IVF culture media [27,38,39]. Our results are in line with those of Karagene et al. [33] and Papayannis et al. [35],where no effect on blastocyst development was observed; however, reduced blastulation rates were demonstrated in other studies [36,37]. To interpret the effect of cytokine integration into IVF culture media, a hypothesis relates to suboptimal and non-physiological conditions, such as the absence of sufficient protein [32,37] or any other poor prognostic factor, as demonstrated by Chu et al. [31], who showed that GM-CSF supplementation increased the biochemical pregnancy, cleavage, and blastocyst formation rates in a subgroup of patients over 38 years old.
Our data demonstrated a significant difference in ongoing pregnancy outcomes in a subgroup of 136 patients who had a previous history of RPL. These findings are consistent with those of Sfontouris et al. [40], who found that the inclusion of GM-CSF in embryo culture media improved the pregnancy and implantation rates in patients with multiple unsuccessful IVF attempts. Similar findings were also reported by Ziebe et al. [4], who found that the greatest benefit of GM-CSF culture medium was observed in women with a history of previous miscarriage, suggesting that the effect was more pronounced in this subgroup. Furthermore, our results indicate that in the RPL subgroup, cytokine supplementation with a low HSA concentration of 2.5 mg/mL was the most cost-effective option evaluated. Regarding the CER results for the overall study, based on the total cost analyses, our results demonstrated that the control group (SSCM) with a common HSA concentration (5 mg/mL) was most cost-effective, with a smaller cost per ongoing pregnancy after fresh ICSI-embryo transfer.
In conclusion, our results indicate that SSCM without cytokine supplementation is more cost-effective than cytokine supplementation. More research is needed to identify micro-environmental factors that affect IVF success. IVF culture medium supplementation means adding costs borne by the patients; therefore, we must consider cost-effectiveness and perform the necessary studies before bringing new add-ons into routine clinical practice. Conducting an RCT to evaluate the benefit of using cytokines in RPL patients would appear to be warranted.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Author contributions
Conceptualization: **. Data curation: **. Formal analysis: **. Funding acquisition: **. Methodology: **. Project administration: **. Visualization: **. Writing–original draft: **. Writing–review & editing: **.