Intraovarian platelet-rich plasma administration could improve blastocyst euploidy rates in women undergoing in vitro fertilization
Article information
Abstract
Objective
Platelet-rich plasma (PRP) therapy has received a considerable attention as an adjunct to fertility treatments, especially in women with very low ovarian reserve and premature ovarian insufficiency. Although recent studies have demonstrated that PRP led to improvements in folliculogenesis and biomarkers of ovarian reserve, the effect of intraovarian PRP administration on embryo genetics has not been studied.
Methods
We report a pilot study of patients who had preimplantation genetic testing for aneuploidy (PGT-A) before and then within 3 months following PRP administration. Twelve infertile women with at least one prior failed in vitro fertilization (IVF) cycle underwent ovarian stimulation (cycle 1) with a gentle stimulation protocol and PGT-A performed at the blastocyst stage. Following cycle 1, autologous intraovarian PRP administration was performed. Within 3 months following PRP administration, the patients underwent cycle 2 and produced blastocysts for PGT-A. The percentage of euploid embryos between both cycles was compared.
Results
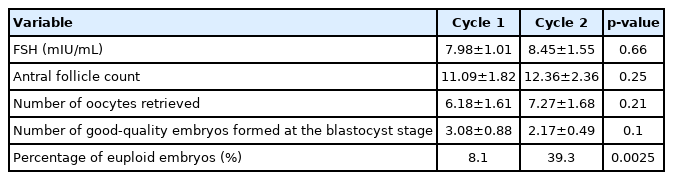
The mean age of all participants was 40.08±1.46 years, and their mean body mass index was 26.18±1.18 kg/m2. The number of good-quality embryos formed at the blastocyst stage was similar between cycle 1 and cycle 2 (3.08±0.88 vs. 2.17±0.49, respectively; p=0.11). Among all patients in cycle 1, 3 of 37 embryos were euploid (8.11%) while in cycle 2, 11 out of 28 embryos were euploid (39.28%, p=0.002). Three clinical pregnancies were noted among this patient group.
Conclusion
This novel study is the first to present an improvement in the embryo euploidy rate following intraovarian PRP application in infertile women with prior failed IVF cycles. The growth factors present in PRP may exhibit a local paracrine effect that could improve meiotic aberrations in human oocytes and thus improve euploidy rates. Whether PRP improves live birth rates and lowers miscarriage rates remains to be determined in large trials.
Introduction
Ovarian aging leads to a decline in both the quantity and quality of oocytes, negatively impacting the formation of genetically normal (euploid) embryos during in vitro fertilization (IVF) treatment and increasing the frequency of miscarriage [1]. The aging process leads to cellular and molecular events such as disturbances in mitochondrial dynamics and mRNA storage, translation, and degradation within the oocytes, all of which contribute to meiotic aberrations [1].
Platelet-rich plasma (PRP) is derived from whole blood, which contains plasma (55%), red blood cells (41%) platelets and white blood cells (4%), by centrifugation and separation of its different components [2]. The centrifugation and separation process leads to the removal of red blood cells and the production of plasma with 5–10 times higher concentrations of growth factors. The platelets present in PRP contain alpha granules that, when activated, release many factors that contribute to growth, cell proliferation, and angiogenesis [3]. The growth factors present in PRP have been shown to play an important role in enhancing collagen synthesis, proliferation of bone cells, fibroblast chemotaxis, macrophage activation, angiogenesis, chemotaxis of immune cells, migration and mitosis of endothelial cells, differentiation of epithelial cells, and cytokine secretion by mesenchymal and epithelial cells [2].
Autologous PRP therapy uses injections of the patient's own concentrated platelets and plasma following a venous blood draw. The theory behind using this modality for treatment stemmed from the natural healing process being the body’s initial response to tissue injury by delivering activated platelets and releasing growth factors. The clinical use of PRP has considerably increased over the last decade, and now includes treatments for musculoskeletal injuries [4-6] arthritis [7], periorbital rejuvenation [8], pancreatic problems [9], dentistry [10], wound healing [11], alopecia [12], and infertility [13]. PRP treatment has recently been used as an adjunct in assisted reproduction technology, in particular, as an intraovarian injection in conjunction with IVF for women who have poor ovarian reserve, premature ovarian insufficiency, and even menopause [14-18].
Recent data have shown that intraovarian PRP treatment led to improvement in markers of ovarian reserve such as serum anti-Müllerian hormone (AMH), a marker of ovarian reserve, and increased oocyte yield with IVF [19]. Although recent studies have demonstrated that PRP led to improvements in folliculogenesis and biomarkers of ovarian reserve, the effect of intraovarian PRP administration on embryo genetics has not been studied, except for one case report that demonstrated qualitative improvement in embryo genetics after intraovarian injection of autologous PRP [20]. We report a pilot study of patients who had preimplantation genetic testing for aneuploidy (PGT-A) before and then within 3 months following intraovarian PRP administration.
METHODS
1. Participants
The participants underwent infertility treatment at Rejuvenating Fertility Center. Infertility was defined as an inability to conceive with unprotected intercourse after 1 year for women aged <35 years, and after 6 months for women aged >35 years. Women with any medical condition that interfered with fertility treatment were excluded from the study. The inclusion criteria were women with at least one previous failed IVF cycle and women who produced fully developed embryos (blastocysts) before and after intraovarian PRP administration (n=12). Each participant underwent two IVF cycles: the first (cycle 1) was followed by intraovarian PRP administration, after which a second IVF cycle (cycle 2) took place within 3 months following the PRP administration. Informed consent was obtained from all patients and the study was approved by the New England Institutional Review Board (NEIRB; No. 120180241).
2. IVF protocols
The IVF cycles 1 and 2 performed in the same participants used similar ovarian stimulation protocols. In brief, in each cycle, after oral contraceptive pill pre-treatment for approximately 2–3 weeks and adequate suppression, minimal/mild ovarian stimulation was started with an extended regimen (from cycle day 3 until the day before triggering) of clomiphene citrate (50 mg/day orally) in conjunction with letrozole (2.5 mg/day orally) with low-dose gonadotropin (75 IU daily) injections (Follistim, Merck, White House Station, NJ, USA; or Gonal F, EMD Serono, Rockland, MA, USA). Hypothalamic-pituitary suppression using a gonadotropin-releasing hormone (GnRH) antagonist was conducted to prevent ovulation. The final maturation of oocytes was induced by a GnRH agonist or by human chorionic gonadotropin trigger when the lead follicle was > 18 mm. The retrieved oocytes were fertilized by intracytoplasmic sperm injection as clinically indicated. All embryos were cultured until the blastocyst stage followed by trophectoderm biopsies for PGT-A and then vitrified to be transferred in a subsequent frozen embryo transfer cycle.
3. Intraovarian PRP administration
PRP was prepared as we previously described [21,22]. Approximately 32 mL of blood was collected from the patient by peripheral venipuncture. The blood sample was placed in a room-temperature centrifuge set to 1,500 ×g for 5 minutes. After centrifugation, the upper layer, corresponding to relatively platelet-poor plasma, was aspirated and discarded, after which the PRP layer was aspirated and placed in a separate tube for a second round of centrifugation, and the lower level corresponding to red blood cells was discarded. The process was repeated a second time. A total of 8 mL of PRP was collected from the tubes, and no activators were used. Under intravenous sedation and transvaginal ultrasound guidance, intraovarian injection of approximately 4 mL of PRP per ovary was performed. The injection was performed in multifocal spots, and diffusion of the PRP in the subcortical layers was achieved by applying 5–7 punctures per ovary transvaginally using a 22-gauge needle and guide. The patients tolerated the procedure well and were discharged home.
4. Statistical analysis
Because the data were normally distributed, we used the paired t-test to compare continuous clinical data between cycles 1 and 2. The chi-square test was used to compare the proportion of euploid embryos between cycles 1 and 2. The statistical analysis was conducted using GraphPad Prism statistical software (GraphPad Software, San Diego, CA, USA), and a p-value of <0.05 was considered statistically significant.
Results
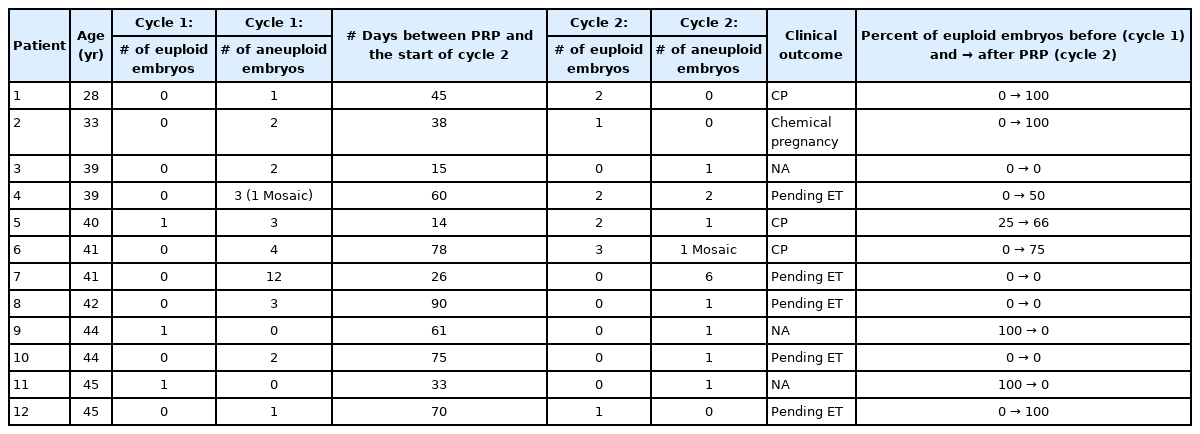
The mean age of all participants was 40.08±1.46 years, and their mean body mass index was 26.18±1.18 kg/m2. When comparing clinical data between cycle 1 and cycle 2, statistically significance differences were not found for serum follicle-stimulating hormone levels (7.98±1.01 mIU/mL vs. 8.45±1.55 mIU/mL, respectively; p=0.66), the antral follicle count calculated by transvaginal ultrasound (11.09±1.82 vs. 12.36±2.36, respectively; p=0.25), the number of oocytes collected (6.18±1.61 vs. 7.27±1.68, respectively; p=0.21), and the number of good-quality embryos formed at the blastocyst stage (3.08±0.88 vs. 2.17±0.49, respectively; p=0.11) (Table 1). Because we used exactly the same protocol for gonadotropins before and after PRP, there was no significant difference in the dose of medications used between cycle 1 and cycle 2 (p>0.05). Among all participants, 3 out of clinical pregnancies were noted among this patient group 1 (8.11%), while 11 out of 28 embryos were euploid in cycle 2 (39.28%, p=0.002). Table 2 shows the individual results for each participant. Three clinical pregnancies were noted among the outcomes of this patient group. The remainder of the patients are either still banking more euploid embryos or in the process of preparing for embryo transfer.
Discussion
For many older infertile women with low ovarian reserve, the production of an euploid embryo is a major challenge along their journey [23]. Here, we present, to our knowledge, the first case series comparing IVF euploidy rates pre-PRP and post-PRP (within 3 months following the PRP procedure). The autologous PRP is known to contain cytokines, chemokines, and growth factors including platelet-derived growth factor, stromal cell derived factor 1, and hepatocyte growth factor [24]. These molecular signals are known to initiate the recruitment, proliferation, and activation of fibroblasts, neutrophils, monocytes, which are expected to regulate angiogenesis and tissue perfusion which might be an independent way to achieve ooplasm improvement within the adult human ovary [19].
Placing autologous cytokines within ovarian tissue may facilitate the production of higher AMH levels by granulosa cells and improve blastocyst ploidy. One possible mechanism is that any new follicles recruited and good quality oocytes obtained after the intraovarian injection of these growth factors have always resided in the ovaries, but are then stimulated by the PRP administration [25]. Another mechanism could be that the platelet growth factors present in the PRP activate, by supplying molecular signals, the existing ovarian stem cells to differentiate into de novo oocytes [2]. In vitro studies demonstrating the effect of PRP on the growth and survival of isolated early human follicles tend to support such theories, as the development and survival rates of preantral follicles in PRP-supplemented culture media have been found to be significantly higher than in media without PRP supplementation, as demonstrated in a dose-dependent manner with both fresh and vitrified ovarian samples [25]. Finally, the oocytes of older women have aberrant meiotic events and impaired fertilization, resulting in poor embryonic development, partly due to altered mitochondrial number and function [26]. Studies have shown that aneuploid embryos have relatively high mitochondrial DNA copy numbers [27]. It is plausible that PRP improves ooplasm quality by altering the mitochondria, leading to improvements in meiosis and thus resulting in ploidy rescue of the embryos.
The limitations of this case series include a small sample size and the lack of a control group, such a group would have been women who underwent ovarian puncture without the injection of PRP, since mechanical puncture of the ovaries could have an effect on ovarian function. Even though all the patients in this reported a history of prior failed IVF, other limitations include the wide range in the ages of patients (from 28 to 45 years old) and the lack of complete pregnancy outcomes in all participants because many of them are still trying to bank more euploid embryos.
In summary, there is a clear need for well-designed studies pertaining to the effect of the commonly used intraovarian PRP administration in women who struggle to form euploid embryos and who ultimately resort to the use of donor oocytes. Some investigators have explored the ovarian germline stem cell niche and its probable regulatory mechanisms with the hope of yielding valuable insights for the treatment of ovarian aging [28]. Investigations related to the effect of PRP on ovarian stem cells are likely to clarify the signaling pathways involved in de novo oocyte replenishment and follicular development, potentially helping older women with abnormal embryo genetics [29].
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Author contributions
Conceptualization: all authors. Data curation: ZM. Formal analysis: ZM, SS. Methodology: ZM, SS. Writing–original draft: ZM. Writing–review & editing: all authors.