Effect of endometrial cell-conditioned medium and platelet-rich plasma on the developmental competence of mouse preantral follicles: An in vitro study
Article information
Abstract
Objective
The aim of this study was to evaluate the impacts of platelet-rich plasma (PRP) and conditioned medium (CM) derived from endometrial stromal cells on mouse preantral follicle culture in a two-dimensional system to produce competent mature oocytes for fertilization.
Methods
In total, 240 preantral follicles were isolated from female mouse ovarian tissue and divided into four groups. The preantral follicles were isolated three times for each group and then cultured, respectively, in the presence of alpha minimum essential medium (control), PRP, CM, and PRP+CM. The in vitro growth, in vitro maturation, and cleavage percentage of the preantral follicles were investigated. Immunocytochemistry (IHC) was also conducted to monitor the meiotic progression of the oocytes. Additionally, the mRNA expression levels of the two folliculogenesis-related genes (Gdf9 and Bmp15) and two apoptosis-related genes (Bcl2 and Bax) were investigated using real-time polymerase chain reaction.
Results
In the PRP, CM, and PRP+CM groups, the preantral follicle maturation (evaluated by identifying polar bodies) were greater than the control group. The cleavage rate in the CM, and PRP+CM groups were also greater than the control group. IHC analysis demonstrated that in each treatment group, meiotic spindle was normal. In the PRP+CM group, the gene expression levels of Bmp15, Gdf9, and Bcl2 were greater than in the other groups. The Bax gene was more strongly expressed in the PRP and control groups than in the other groups.
Conclusion
Overall, the present study suggests that the combination of CM and PRP can effectively increase the growth and cleavage rate of mouse preantral follicles in vitro.
Introduction
Fertility preservation strategies, which involve cryopreservation of the oocyte and embryo, can help achieve successful pregnancy; however, some obstacles exist. For example, these methods require time to stimulate ovulation, delaying the therapeutic process for patients with cancer [1]. Furthermore, substantial concerns exist regarding cancer recurrence after ovarian tissue cryopreservation and autotransplantation due to the possibility of malignant cell contamination [2-4]. In this context, in vitro follicle culture may be superior to the mentioned methods because it does not increase the risk of cancer recurrence and does not delay the treatment of cancer patients [2,5-7].
Advanced technologies to facilitate oocyte growth and development in culture enable experimental research on the mechanisms regulating oocyte development, in addition to clinical applications [8]. Due to ethical and practical limitations, the application of human research to evaluate oocyte maturation has been limited and requires the use of animal studies. Some of these issues can be addressed by utilizing murine follicles, which are easily accessible [9,10]. In the last two decades, preantral follicle cultures have provided many oocytes for embryo production [11]. A two-step culture method was discovered to create competent oocytes for maturation, fertilization, and development into live offspring from newborn mouse primordial follicles [12,13]. Follicle culture must be optimized to improve oocyte maturation and quality regarding genetic components such as meiotic spindles [5]. Furthermore, coculture with uterine epithelial cells by promoting oocyte maturation to meiosis II can increase the maturation of germinal vesicle oocytes [14].
Compared to the co-culture of somatic cells, using conditioned medium (CM) is superior as it avoids the close contact of certain somatic cells, which can result in physiological impairment and/or the reinitiation of latency in follicles. Furthermore, the CM can be retained for later use [15]. Some studies have indicated greater viability and larger diameter of follicles cultured with CM than control medium, indicating that CM could improve follicle culture [16]. Sources of CM include endometrial cells, which secrete various factors involved in cell proliferation and migration [17-19]. CM derived from human endometrial cell lines produces several cytokines and growth factors, including interleukin 6, platelet-derived growth factor, and interleukin 10 [17-19]. Interestingly, these factors play an important role in the modulation of ovarian function, including follicular growth, maturation, and theca cell proliferation [20-22]. Platelet-rich plasma (PRP), an accumulation of platelets suspended in plasma, is another source that can promote the viability and growth of various cell lines, likely due to the presence of growth factors [23-26]. Some of these growth factors, such as epidermal growth factor (EGF), connective tissue growth factor, and transforming growth factor beta (TGF-β), are involved in follicular development and oocyte maturation [27,28]. Several studies have shown that PRP factors benefit follicle growth and the survival of primordial follicles in in vivo and in vitro three-dimensional culture [27,29]. Thus, PRP and CM can affect the growth, maturation, and viability of preantral follicles.
In ovarian tissue culture, preantral follicles typically do not progress to meiosis II [11]. In contrast, the in vitro culture of preantral follicles is promising for the evaluation of oocyte maturation and comparative analyses of factors such as culture medium in in vitro follicular culture [30]. Therefore, this investigation was carried out to assess the influences of endometrial stromal cell-derived PRP and CM on in vitro growth, in vitro maturation (IVM), cell viability, and the cleavage rate of mouse preantral follicle culture in a two-dimensional system.
Methods
1. Animals
In this experimental animal study, female National Medical Research Institute (BALB/c) mice were purchased from the Royan Institute in Iran for the isolation of ovarian tissue. Additionally, 20 adult male BALB/c mice were purchased for sperm collection. The mice were maintained in the animal house under light- and temperature-controlled conditions (12 hours of light and 12 hours of darkness; 21°–24°C and 30%–60% humidity), and fresh food and water were provided. The animals were kept and used according to the standards of the Animal Ethics Committee. The experimental procedure of this study was approved by the Ethics Committee of Shiraz University of Medical Sciences (reference No. IR. Sums.REC.1398.688).
2. Ovarian tissue isolation
The female mice were sacrificed with carbon dioxide (CO2), and the ovaries were transferred to a HEPES (N-2-hydroxyethyl-piperazine-N’-2-ethanesulfonic acid)-based potassium simplex optimization medium (KSOM). The preantral follicles were isolated from the ovarian tissue and were cultured in four treatment groups: alpha minimum essential medium FBS (control), CM, PRP, and PRP+CM.
3. Preantral follicle isolation
From the fresh ovarian tissues, 240 preantral follicles were mechanically isolated. These preantral follicles were isolated three times for each group. To accomplish this, preantral follicles were separated in the KSOM medium (at 37°C) under a stereomicroscope using a 26-gauge needle. The isolated preantral follicles were selected if they (1) were normal preantral follicles with two or more compact granulosa cell layers and some adjoining theca cells and (2) had a clear, round oocyte in the center of the preantral follicles. The isolated preantral follicles were cultured in FBS (control), CM, PRP, or PRP+CM media.
4. PRP collection
Blood samples from female BALB/c mice were obtained via cardiac puncture and poured into a tube containing an anticoagulant agent (3.2% sodium citrate). The blood samples were then centrifuged at 250 ×g for 10 minutes at 20°C. The supernatant was transferred to another tube and then centrifuged at 2,000 ×g for 15 minutes. The two upper layers were removed, and the residual plasma, including precipitated platelets, was considered the PRP. Finally, the obtained clots were centrifuged at 3,000 ×g for 5 minutes at 4°C; the platelet fragments were separated and kept at −20ºC for further experiments [27,29]. To count the platelets in the obtained PRP, the Sysmex XT-1600I system (Sysmex, Kobe, Japan) was utilized and revealed an average of 2,380 ×103 platelets/mL.
5. Isolation and culture of endometrial stromal cells
In this investigation, female BALB/c mice were purchased from the Royan Institute in Iran. The animals in the estrus cycle were euthanized using narcosis induced by CO2. Using sterile surgical instruments, the abdominal region was opened, and the intestines were pushed aside to observe both uterine horns. The uterine horns were isolated at the most distal portion of the Fallopian tube. Then, the uterine horns were dissected from the Fallopian tube, and surrounding connective and adipose tissues were removed. Afterward, the endometrial samples were obtained and washed in PBS [30-32].
Next, the samples were minced and incubated at 37°C with 0.2% HBSS with 15 mM HEPES, gentamicin (10 μg/mL), 2% (v/v) fetal calf serum, and 1×antibiotic-antimycotic solution (fungizone [2.5 μg/mL], streptomycin [100 μg/mL], and penicillin [100 IU/mL]) for 30 minutes with shaking (50 rpm). Next, the samples were isolated is according to Clercq De protocol [10].
1) Endometrial stromal cell-derived CM
When cells reached approximately 80% confluence, daily changes of medium were carried out for 24 hours; the mixture used included serum-free DMEM:F12 (1:1) medium added to insulin (10 μg/mL), selenium (6.7 ng/mL), transferrin (5.5 μg/mL), hydrocortisone (5 μg/mL), penicillin (100 IU/mL), fungizone (2.5 μg/mL), streptomycin (100 μg/mL), and gentamicin (10 μg/mL) [32].
6. 2,5-diphenyl-2H-tetrazolium bromide (MTT) assay
The survival rate of the preantral follicles was analyzed to determine the effective dose of PRP and endometrial cell-derived CM using the MTT assay. For this purpose, 60 preantral follicles were collected and randomly divided into six groups, then seeded in a 96-well plate. Each group contained 10 preantral follicles in an α-MEM culture medium, comprising 1% insulin-transferrin-selenium (ITS), ascorbic acid (50 µg/mL), penicillin-streptomycin (50 µg/mL), follicle-stimulating hormone (FSH; 100 mIU/mL), and luteinizing hormone (LH; 10 mIU/mL). After 24 hours, fresh media (200 µL) containing concentrations of either 5% PRP, 10% PRP, 5% CM, or 10% CM was added to four of the groups; the remaining two groups were not treated. The plates were incubated for 48 hours. A fresh medium (200 μL) containing an MTT agent (5 mg/mL in medium) was substituted and the plates incubated at 37°C for 4 hours. Then, dimethyl sulfoxide (50 μL) was substituted, and the plates were incubated at 37°C for 30 minutes. Subsequently, the optical densities of all wells were evaluated using an enzyme-linked immunoassay reader at a wavelength of 570 nm [29-33].
7. In vitro growth
Penicillin-streptomycin (50 µg/mL), 1% ITS, FSH (100 mIU/mL), LH (10 mIU/mL), and ascorbic acid (50 µg/mL). Moreover, each group was complemented with either 10% FBS (control), 10% PRP, 10% CM, and 10% PRP+10% CM, then incubated for 8 days at 37°C. The medium was substituted once every 2 days [6,11,34].
8. In vitro maturation
After 8 days of culture, the follicles in the four groups were transferred to the culture medium supplemented with 1.5 IU/ml HCG and then incubated at 37°C. After 24 hours, the existence of polar bodies and the expansion of cumulus cells were evaluated. Mature oocytes were identified based on the identification of polar bodies via stereomicroscopy. Next, the mature follicles were assessed regarding the presence of the meiotic spindle (through fluorescence microscopy), relevant gene expression, and in vitro fertilization (IVF) [6].
9. In vitro fertilization
The oocytes accompanied by polar bodies in meiosis II were incubated with capacitated spermatozoa isolated from male BALB/c mice. Then, the oocytes were separated and transferred to a global medium (20 µL) under mineral oil at 37°C and 5% CO2, and then the IVF rate in the cleavage stage was assessed [35].
10. Immunocytochemistry
The obtained meiosis II oocytes were fixed with a microtubule-stabilizing buffer containing Triton X-100 (0.1%), formaldehyde (3.7%), dithiothreitol (1 mM), Taxol (1 mM), deuterium oxide (50%), and aprotinin (0.01%). The oocytes were added to a phosphate-buffered saline blocking solution consisting of normal goat serum (2%), bovine serum albumin (1%), sodium azide (0.2%), powdered milk (0.2%), Triton X-100 (0.01%), and glycine (0.1 M) and were stored at 4°C. Chromatin was detected using the triple staining method. To do this, the oocytes were incubated for 4 hours in a mixture of mouse monoclonal alpha and beta anti-tubulins (Thermo Fisher Scientific, Waltham, MA, USA) at 1:500 final dilution and then were washed with a phosphate-buffered saline blocking solution and incubated in a 1:400 dilution of Alexa 488 goat anti-rabbit IgG (1:500) (Thermo Fisher Scientific) and rhodamine phalloidin (1:200) (Sigma, St. Louis, MS, USA) for 3 hours at 37°C. The oocytes were transferred to a glycerol-based medium with propidium iodide (1 ng/mL) on posts of Vaseline (1 mm) to stop the oocyte compression. A Labomed device (Labomed Inc., Culver City, CA, USA) was utilized for fluorescent microscopy, and photos were taken with a water immersion objective with stimulation lines at 400 nm (an argon laser for anti-tubulin) [11].
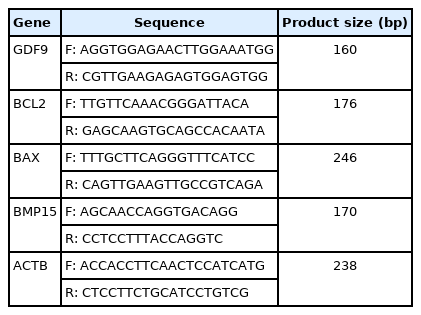
11. Real-time quantitative polymerase chain reaction
The extraction of total RNA from the preantral follicle after 9 days was performed using an RNA isolation kit according to the manufacturer's instructions. Next, the synthesis of complementary DNA (cDNA) was conducted via reverse transcription. The mRNA expression levels of the BAX, BCL2, BMP15, and GDF9 genes were investigated using real-time quantitative PCR (RT-qPCR). The primer sequences used are presented in Table 1. The RT-qPCR (Applied Biosystems, Waltham, MA, USA) was carried out in a 10-μL volume including PCR pre-Mix (5 μL RR820L; Takara Bio Inc., Kusatsu, Shiga, Japan), forward/reverse primers (5 μM), and cDNA (1 μL) as follows: initial denaturation (1 minute at 94°C), denaturation (10 seconds at 94°C), annealing (30 seconds at 59°C), and extension (20 seconds at 72°C). The mRNA levels of the β-actin gene were evaluated as the endogenous control. Calculations were performed using the 2−ΔCt (Livak) formula [36].
12. Data analysis
Statistical analyses were carried out using GraphPad Prism statistical software (GraphPad Software, San Diego, CA, USA). The differences between groups were measured using the Tukey test, and statistical significance between more than two groups was investigated using the analysis of variance test. All obtained data were reported as mean±standard deviation. A p-value of <0.05 was considered to indicate statistical significance.
Results
1. Oocyte maturation
Preantral follicles with two or more compact granulosa cell layers were isolated three times for each group (Figure 1). The oocyte maturation rates in the four groups are presented in Figure 2. The maturation rate (based on the observation of polar bodies) of the group treated with CM was significantly greater than that of the control group (p<0.01). Oocyte maturation was also dramatically greater in the PRP group than in the control group (p<0.05). Finally, the oocyte maturation rate was significantly elevated in the PRP+CM group relative to the control group (p<0.01).

(A-D) Isolated preantral follicles with granulosa cell layers. (E-H) Mature oocytes (MII). (I-L) Cleaved oocytes. (A, E, I) The platelet-rich plasma (PRP). (B, F, J) The conditioned medium (CM). (C, G, K) The PRP+CM group. (D, H, L) The alpha minimum essential medium (α-MEM). Original magnification, ×400; scale bar, 100 μm. GV, germinal vesicle; MII, meiosis II; O, oocyte; G, granulosa cells; P, polar body.
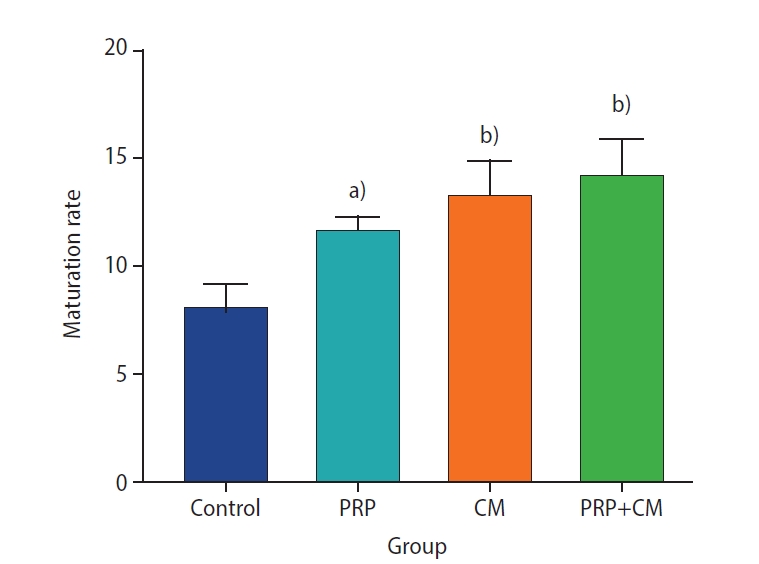
The maturation rate of oocytes (based on the observation of polar bodies) after in vitro growth (7 days) and in vitro maturation (24 hours). The level of oocyte maturation rate were significantly greater in all treatment groups than in the control group. PRP, platelet-rich plasma; CM, conditioned medium. Treatment group vs. control group, a)p<0.05; b)p<0.01.
2. The oocyte cleavage rate
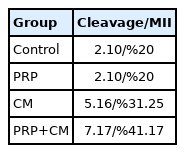
The oocyte cleavage rate for each group is shown in Table 2. Our findings indicated that the cleavage percentage in the CM group was %31.25, in the PRP group was %20, and finally in the treated group with both CM and PRP was %41.17.
3. Preantral follicle viability
The viability of preantral follicles in the groups, analyzed to determine the effective dosage of PRP and CM, is exhibited in Figure 3. Our findings demonstrate that the viability of preantral follicles treated with 10% PRP was significantly greater than the viability in the groups treated with other concentrations of PRP (10% PRP vs. 5% PRP, p<0.05; 10% PRP vs. 0% PRP, p<0.05). Furthermore, the viability of the group exposed to 10% CM was significantly greater than the viability of those exposed to other CM concentrations (10% CM vs. 5% CM, p<0.05; 10% CM vs. 0% CM, p<0.01).
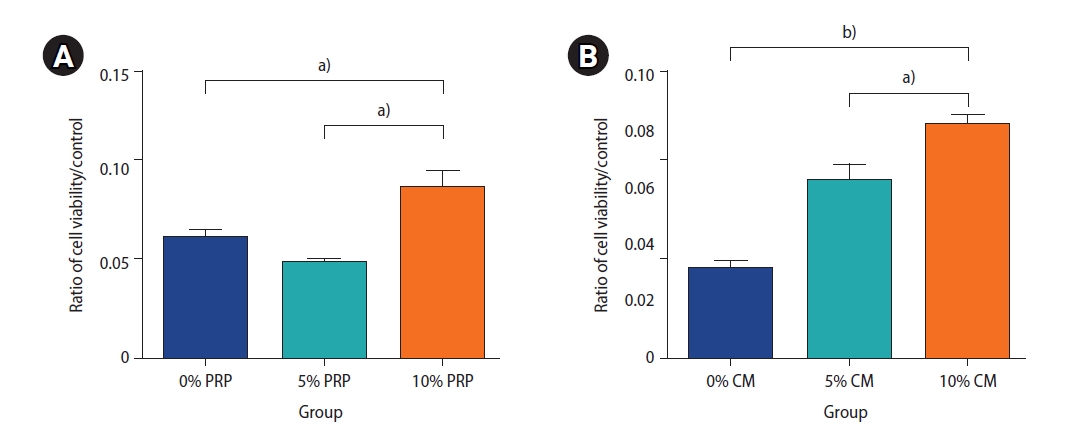
The survival rate of the preantral follicles was analyzed to determine the effective dose of platelet-rich plasma (PRP, A) and endometrial cell-derived conditioned medium (CM, B) using the MTT assay after 48 hours. The samples contained different concentrations of PRP and CM: 0% PRP, 5% PRP, and 10% PRP and 0% CM, 5% CM, and 10% CM. The preantral follicle survival rate was obtained three times for each group. a)p<0.05, b)p<0.01.
4. Meiotic spindle positioning
Immunocytochemistry analysis indicated the presence of meiotic spindle, meaning that these oocytes had proceeded in meiosis and attained meiotic competence. We found that the spindles were oriented parallel to the oocyte surface in the treatment groups (PRP, CM, and PRP+CM) (Figures 4 and 5).

Fluorescent images of preantral follicle culture, including the meiotic spindle positioning in the mouse oocyte. The condensed cumulus is detached from the meiosis II oocytes. The meiosis II oocyte is shown with first polar body disjunction following in vitro growth and in vitro maturation, as well as a meiotic spindle (green) and chromosomes (red) after immunofluorescence staining. The spindles with spired poles are located in the oocyte cortex. An extra meiosis II with more common wide spindle poles and chromosome adjustment is depicted. Original magnification, ×400; scale bar, 100 μm. The meiotic spindle was oriented parallel to the oocyte surface in the treatment groups (platelet-rich plasma [PRP], conditioned medium [CM], and PRP+CM).
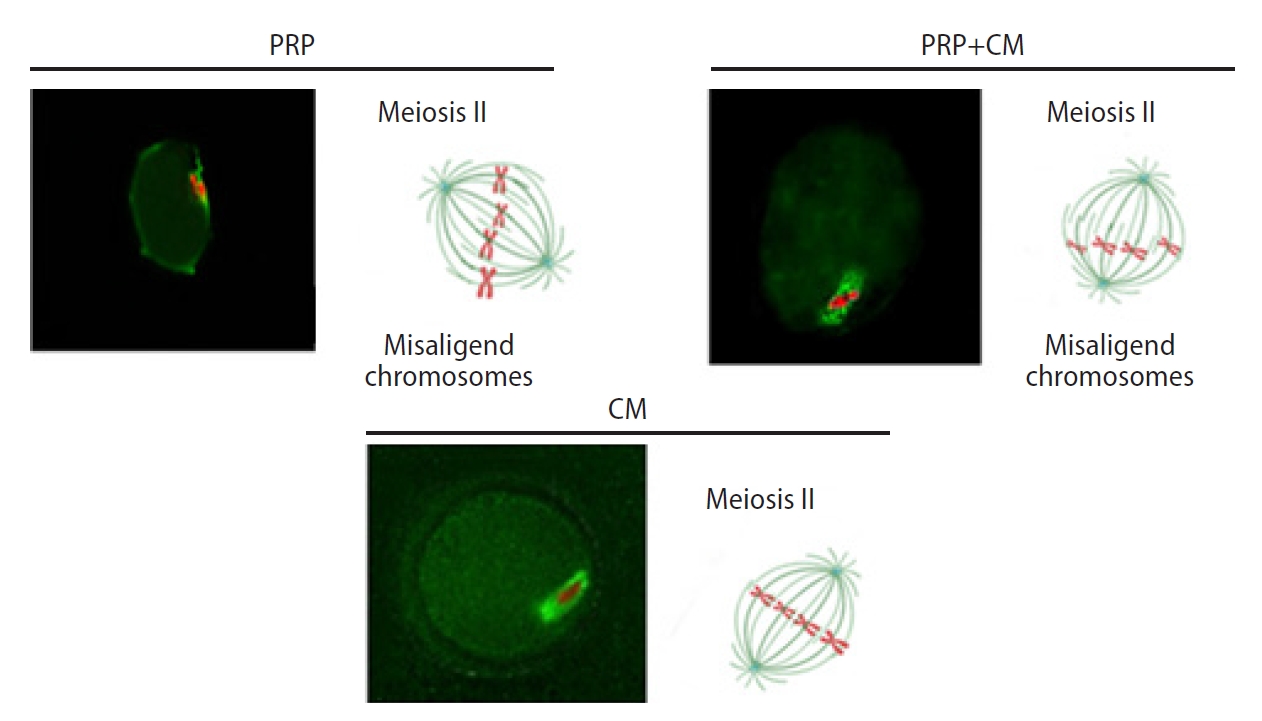
Meiotic spindle migration. The spindles are located in the oocyte cortex. The migration of the meiotic spindles from the center of the oocytes to the cortex occurred. The outer circle indicates the zona pellucida; microtubules are shown in green and chromosomes in red. PRP, platelet-rich plasma; CM, conditioned medium.
5. Gene expression
The expression levels of the BAX, BCL2, BMP15, and GDF9 genes of the preantral follicles were cultured in the four groups are shown in Figure 6. The findings indicated that the expression levels of Bmp15, Gdf9, and Bcl2 were significantly upregulated in the preantral follicles of the PRP+CM group compared with the other groups. In contrast, the Bax gene was expressed most strongly in the control and PRP groups.
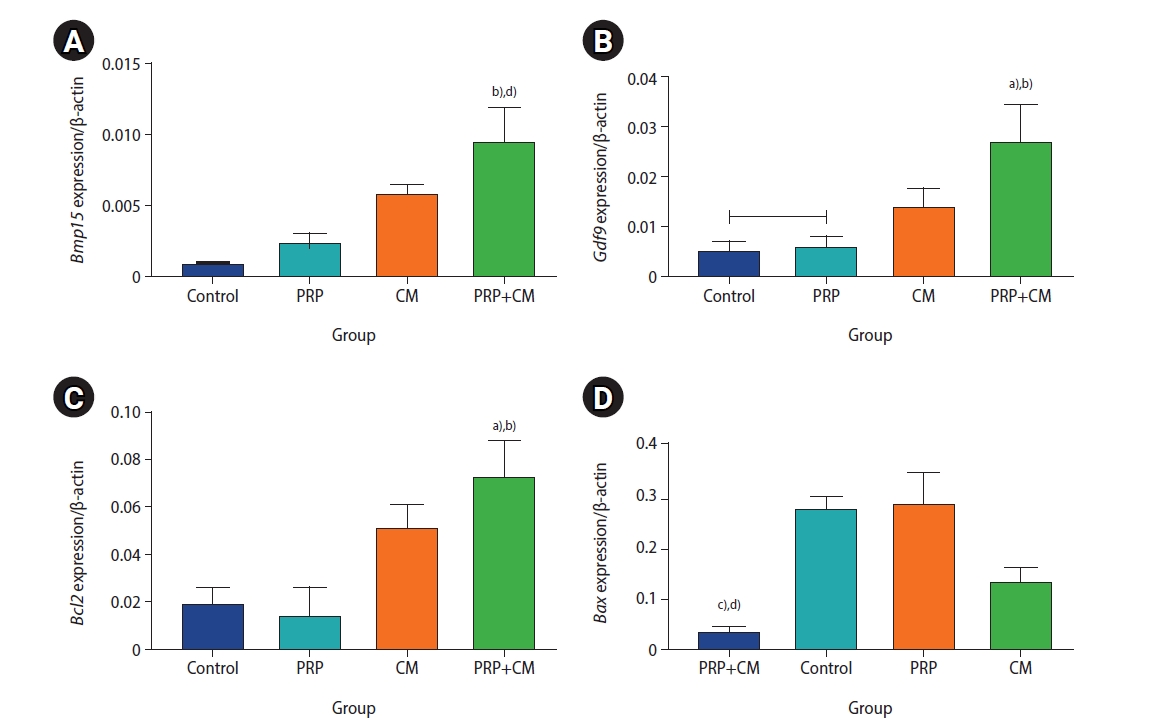
mRNA expression levels of the Bmp15 (A), Gdf9 (B), Bcl2 (C), and Bax (D) genes in the meiosis II oocytes in the four groups. The mRNA expression level in the preantral follicles was obtained three times for each group. PRP, platelet-rich plasma; CM, conditioned medium. PRP+CM group vs. control group: a)p<0.05, d)p<0.01; PRP+CM group vs. PRP group: b)p<0.05, c)p<0.01.
Discussion
In vitro, preantral follicle culture is a key method of fertility preservation for either women of reproductive age or prepubertal girls who lack hormonal stimulation or are at risk of cancer. Therefore, the enhancement of preantral follicle culture medium may help obtain a better embryo and improve the odds of successful pregnancy [11]. Numerous approaches to in vitro culture have been introduced for female gamete growth; however, the production of competent oocytes is still challenging [5]. Hence, in this work, the development of mouse preantral follicles using CM derived from endometrial cells and PRP was investigated for the first time. Because CM and PRP contain different cytokine and growth factors, they can impact ovarian follicle growth [18-19,27,29]. Thus, in this project, the effects of CM, PRP, and PRP+CM were evaluated on in vitro oocyte development, fertilization potential, quality of the meiotic spindle, and expression of related genes (BPM15, GDF9, BAX, and BCL-2). Our study revealed that the maturation rate of oocytes in all treatment groups (PRP, CM, and PRP+CM) was significantly greater than in the control group.
The impacts of PRP and CM on preantral follicle growth have been posited to be due to different growth factors, such as fibroblast growth factor, supporting the early stage of preantral follicle development [37]. In addition, previous studies have reported the stimulation of oocyte maturation and cumulus cell proliferation by EGF [37]. The investigation of Hosseini and colleagues indicated that PRP can improve the growth and viability of human preantral follicles in vitro [29]. Similarly, Adib et al. [35] declared that CM originating from human cumulus cells could enhance oocyte growth and maturation in vitro. However, in the present study, the maturation and cleavage rate in the PRP group were lower than in the CM and PRP+CM groups.
Another result was that meiotic spindles of the oocytes had proceeded to meiosis II in the treatment groups. Along these lines, researchers have posited that some growth factors, such as fibroblast growth factor, EGF, and TGF-β, play a substantial role in the resumption of meiosis in oocytes [38-40]. The spindles were oriented parallel to the oocyte surface in the CM, PRP, and PRP+CM groups, like the orientation of meiosis II spindles parallel to the oocyte cortex surface in the mice studied by McNally [41].
Molecular findings revealed that the expression levels of the BMP15, GDF9, and BCL2 genes were upregulated in the preantral follicles of the PRP+CM group relative to the other groups. In contrast, the BAX gene was less strongly expressed in this group than in the others, whereas BAX was most strongly expressed in the presence of PRP. BCL-2 and BAX are among the most important genes involved in apoptosis. The BCL-2 gene-encoded product inhibits apoptosis and promotes increased cell survival. In contrast, the BAX gene-encoded product induces apoptosis and causes increased cell death [42]. Increased expression of the BCL-2 gene can potentiate the viability and growth of preantral follicles in the presence of PRP+CM.
Likewise, downregulation of the BAX gene in the cultured preantral follicles exposed to PRP+CM can decrease the apoptosis rate. In the current study, we found greater expression of BAX in the PRP group than in the CM and PRP+CM groups. PRP has been reported to be involved in the formation of reactive oxygen species, leading to increased BAX expression [43,44]. In addition, the BMP15 and GDF9 genes, secreted by oocytes, can regulate granulosa cell proliferation and differentiation and promote preantral follicle growth [45]. Previous studies have reported that BMP15 present in the follicular fluid of mature follicles, which may be synthesized by the oocytes of these follicles, regulates cumulus cell expansion. Moreover, evidence suggests that GDF9 is found in mature oocytes [46]. The strong expression of BMP15 and GDF9 can explain the relatively high developmental competence of the preantral follicles in the PRP+CM group. Due to the different effects of PRP reported in our work and others on ovarian follicle culture, which may lead to different impacts on the viability of ovarian follicles, further investigation is warranted on reactive oxygen species in PRP and culture media.
This study suggests that the combination of PRP and CM can increase the growth and cleavage rate of mouse early preantral follicles, as well as the expression of related genes. Additionally, the expression of the BAX gene was increased in the PRP group relative to other groups. However, additional, large in vitro experimental investigations, particularly oxidative assessments of PRP in follicle culture, are required to validate these findings.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Author contributions
Data curation: NT. Formal analysis: NT, FD, GM. Funding acquisition: FD, FA. Methodology: NZF. Writing–original draft: NT. Writing–review & editing: FD.
Acknowledgements
This article was extracted from the PhD thesis written by Neda Taghizabet. The research was performed at the Men’s Health and Reproductive Health Research Center at Shahid Beheshti University of Medical Sciences and the Experimental and Comparative Medical Center, Anatomy Department, Shiraz University of Medical Sciences in Shiraz, Iran.