Sperm DNA fragmentation in consecutive ejaculates from patients with cancer for sperm cryopreservation
Article information
Abstract
Objective
This prospective consecutive study investigated the variation in sperm DNA fragmentation (SDF) in multiple semen samples from patients with cancer.
Methods
Eighty-one patients with various cancers underwent multiple semen collections on 3 consecutive days for sperm cryopreservation prior to cancer treatment. A commercial Halosperm kit was used to measure SDF. Within- and between-subject coefficients of variation were estimated via random-effects analysis of variance to assess the consistency of semen parameters and SDF. Intraclass correlation coefficients (ICCs) were calculated to assess the magnitude of the between-subject component of variance relative to the total variance.
Results
The volume of semen in the day-2 and day-3 samples was significantly lower compared with the day-1 sample. Most parameters showed high ICC values, suggesting that within-subject fluctuations were small relative to the between-subject variability. The highest ICC values were identified for the SDF (ICC, 0.68; 95% confidence interval [CI], 0.45–0.84) and semen volume (ICC, 0.67; 95% CI, 0.45–0.84).
Conclusion
Our findings showed that repeated ejaculates from patients with cancer had stable SDF levels.
Introduction
The number of male cancer survivors of reproductive age has been steadily increasing, and concerns for the quality of life of patients with cancer, including fertility preservation, have received widespread attention. Sperm cryopreservation has been strongly recommended before cancer treatment since sperm quality may decrease posttreatment [1,2]. To obtain the desired number of sperm samples for cryopreservation, male patients are required to ejaculate multiple times within several days.
The World Health Organization (WHO) recommends that semen be collected after abstinence for 3–7 days [3]. To collect enough samples, the American Society of Clinical Oncology guideline recommends that sperm banking be performed quickly, at 24-hour intervals [4]. To the best of our knowledge, only one study has evaluated the quality of semen in male patients with cancer who underwent several sessions of ejaculation within a short period [5]. When analyzing the consistency of conventional semen parameters, repeated ejaculates did not show significant variation in semen quality over a maximum of 5 consecutive days. However, we cannot conclude that the sperm is completely normal because conventional semen parameters do not include all functions of sperm.
In current clinical practice, the evaluation of male fertility is largely dependent on conventional semen analysis. However, conventional semen analysis can be unreliable for predicting in vitro fertilization (IVF) outcomes. To overcome these limitations, the use of sperm DNA fragmentation (SDF) analysis has gained increasing popularity. Recent studies [6-8] have demonstrated that SDF levels have a significant association with IVF outcomes. In IVF/intracytoplasmic sperm injection (ICSI) cycles, a high SDF level was shown to be associated with low embryo formation rates [6]. A high SDF level was also associated with a high miscarriage rate [7]. A meta-analysis including 13 prospective studies showed that male partners with a history of recurrent pregnancy loss have significantly higher levels of SDF compared with fertile control participants [8].
There are various techniques for measuring SDF, including the terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling assay, sperm chromatin structure assay, Comet assay, and sperm chromatin dispersion (SCD) assay. The SCD assay is widely used because it is simple, quick, and highly reproducible [9]. Several studies [10-12] have reported on the consistency of semen parameters using within-subject coefficients of variation (CVw). However, variation in the semen quality of repeated ejaculates related to SDF has never been investigated, especially in patients with cancer. In the present study, we used the SCD assay to analyze the variation of conventional semen parameters as well as SDF in patients with cancer who visited our sperm bank clinic before cancer treatment.
Methods
1. Subjects
Eighty-one patients with various cancers underwent one or multiple semen collections for sperm cryopreservation between 2016 and 2017 at the Seoul National University Bundang Hospital. Germ cell tumors (16 patients) and lymphomas (16 patients) were the two most common cancers, followed by gastrointestinal cancer (15 patients) and leukemia (7 patients). The mean age of the patients at the time of semen collection was 27.5±7.5 years (range, 14–42 years) and most were not married. None of the patients had received chemotherapy before semen collection. The Institutional Review Board of the Seoul National University Bundang Hospital approved the use of individual data from patients’ medical records (No. B-1403-242-102). All participants provided written informed consent.
2. Laboratory analysis
Semen collections were repeated one to five times (mean, 2.6±0.8) for each patient within a maximum 5 days. All semen samples were obtained in sterile containers by masturbation. After liquefaction for 30 minutes at room temperature (RT), routine sperm quality was assessed via a computer-assisted semen analysis system (SAIS-PLUS 10.1; Medical Supply, Seoul, Korea) within 1 hour of collection. The evaluated semen parameters were semen volume (mL), sperm concentration (×106/mL), total sperm count (semen volume×sperm concentration), progressive motility (%), and total motile count (TMC) (semen volume×sperm concentration×progressive motility/100). To ensure accuracy of the results, a manual assessment was also performed.
For the SCD assay, a Halosperm kit (Halotech DNA, Madrid, Spain) was used, as described previously [13]. The semen samples (25 µL) were mixed with pre-warmed agarose gel and dropped onto slides. The slides were covered with a glass coverslip and kept in a refrigerator for 5 minutes at 4°C to create a microgel with the implanted sperm. The coverslip was then removed and the slides were immersed in a prepared acid solution (80 µL of hydrogen chloride in 10 mL of distilled water) for 7 minutes at RT. The slides were then transferred to the tray with a lysis solution and incubated for 25 minutes at RT. The slides were rinsed with distilled water for 5 minutes, followed by dehydration in increasing concentrations of ethanol (70%, 90%, and 100%, for 2 minutes each). After drying, the slides were stained with Diff-Quik (Baxter Diagnostics Inc., McGaw Park, IL, USA), rinsed under tap water, and air-dried at RT.
Each slide was examined under a light microscope at ×400 magnification, and at least 200 sperms were assessed for halo patterns. Each sperm was categorized as having a large halo, medium halo, small halo, no halo, or degraded. Sperms with a small halo or no halo and degraded sperms were classified as sperms with fragmented DNA. The SDF level was the percentage of sperms with fragmented DNA per total sperms.
3. Data analysis
We initially obtained 172 semen analysis results from 97 male patients. The semen samples collected on the first day were regarded as day-1 (D1) samples. There were 81 D1 samples, 51 day-2 (D2) samples, 20 day-3 (D3) samples, 11 day-4 (D4) samples, and 9 day-5 (D5) samples. Because the D4 and D5 samples were too small, we only analyzed the D1, D2, and D3 samples (81 men, 152 samples). The semen analysis results of the D2 and D3 samples were compared with the results of the D1 samples using the Wilcoxon signed-rank test. Based on our previous study [5], we selected the parameters (volume, concentration, motility, and SDF) that we thought were the most meaningful. The relationship between SDF levels and the other semen parameters was evaluated using linear regression analysis.
The coefficient of variation (CV) was calculated as the square root of the variance component estimate divided by the overall mean and expressed as a percentage (CV=[standard deviation/mean]×100). Correlations between the within-subject standard deviation and individual means were analyzed using the Spearman correlation test. To compare the size of the between-subject coefficient of variance (CVb) to the total (between- and within-subject) component of variance, intraclass correlation coefficients (ICCs) in a two-way random effects model were used. In this setting, the ICC could estimate how strongly repeated measures in the same individual were correlated, thereby providing a measure of the within-subject consistency (stability) of the semen parameters. A high ICC value indicates that within-subject fluctuations were small relative to the between-subject variability. In the present study, the following scale was used to interpret reliability: excellent, >0.75; good, 0.60–0.74; fair, 0.40–0.59; and poor, <0.4.
Results
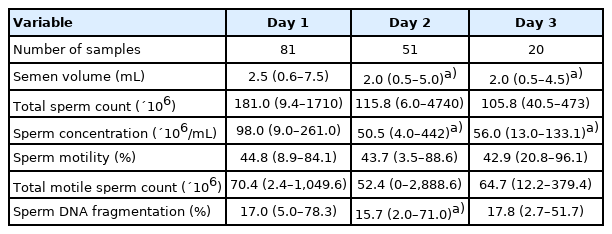
Semen volume, sperm concentration, motility, and SDF levels from the D1 to D3 samples are depicted in Figure 1 as box and whisker plots. As shown in Table 1, semen volume and sperm concentration were significantly reduced in the D2 and D3 samples when compared with the D1 samples. Total sperm count, motility, and TMC were not changed significantly in D1 through D3 samples. The SDF level was significantly reduced in D2 samples only, when compared with D1 samples. Correlations between SDF levels and other semen parameters are shown in Table 2. There was no association between SDF levels and semen volume, sperm concentration, or total sperm count in the D1, D2 and D3 samples. The SDF level had a significant negative relationship with motility in the D1 samples only (r=–0.273, p=0.014).
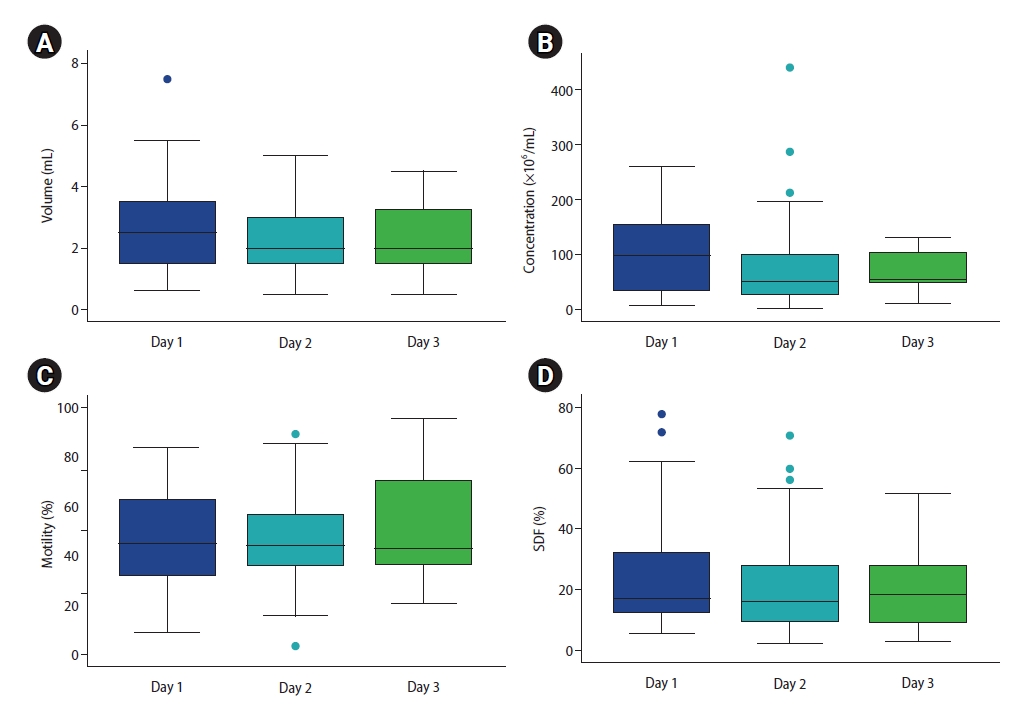
Box whisker plots showing consecutive changes in semen volume (A), sperm concentration (B), sperm motility (C), and sperm DNA fragmentation (SDF; D) levels from day 1 to day 3 semen samples in 81 patients with cancer (number of samples: day 1, 81; day 2, 51; day 3, 20).

Correlation coefficients between sperm DNA fragmentation level and other semen parameters from the first day sample to the third day samples
The CVw, CVb, and ICC values for various semen parameters are presented in Table 3. All analyzed parameters showed higher between-subject variability than within-subject variability. Semen volume and motility demonstrated the smallest degree of variation, both within and between subjects. Semen volume and motility showed the lowest CVw (26.4 and 27.4, respectively), whereas total sperm count and TMC showed the highest CVw (68.3 and 58.0, respectively).
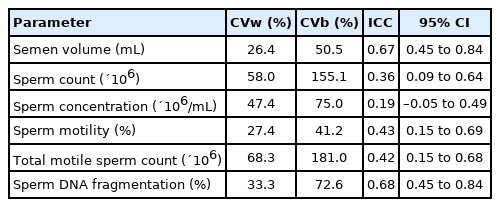
Within-subject coefficients of variation, between-subject coefficients of variation, and intraclass correlation coefficients of semen parameters in three-times ejaculates from 81 men
Semen volume and SDF showed an ICC value with good reliability (between 0.60 and 0.74). The ICC value of SDF was highest (0.68; 95% confidence interval [CI], 0.45–0.84), followed by semen volume (0.67; 95% CI, 0.45–0.84). The ICC values of total sperm count and sperm concentration were less than 0.40.
Discussion
All semen parameters of the D1, D2, and D3 samples were maintained within the normal range according to the 2010 WHO guidelines [3]. This suggested that the presence of various cancers did not significantly affect semen quality in the study group. Although there have been conflicting results, previous studies have shown normal semen analysis of patients with various cancers (except testicular cancer) [14,15]. This study confirmed that repeated ejaculates from patients with a variety of cancers maintained good sperm quality, as we also reported in a previous study [5]. In our data, the median SDF level was 17.0% in D1 samples, 15.7% in D2 samples, and 17.8% in D3 samples. All values were less than 30% (a cutoff point suggested by previous literature [16-18]), which supports the finding that repetitive ejaculates from patients with a variety of cancers maintained relatively stable DNA integrity during the 3 days of sperm collection.
The conventional semen parameters of repetitive ejaculates in the same individual are known to have a wide CV due to high biological variation [12,19]. Therefore, at least two semen samples should be examined after 3-7 days of ejaculatory abstinence to assess the fertility of male partners [3]. In the present study, the CVw ranged from 26.4%–68.3% and the CVb ranged from 41.2%–181.0%. Similar results were observed in our previous study (CVw, 17.2%–51.5%; CVb, 29.6%–146.8%) [5], as well as another study on healthy men [12]. The present results showed that the CVb in all parameters was higher than the CVw, and that sperm concentration showed the highest variation, as reported in previous studies [5,10].
To the best of our knowledge, this is the first report to demonstrate the consistency of SDF during repetitive semen collections. The SDF levels in the present study were much higher in the CVb than the CVw, which suggests that SDF is highly individual. Because SDF levels showed relatively low CVw and high ICC values, the SDF level was a highly reliable parameter among several semen parameters.
There have been several studies analyzing the association between SDF and semen parameters [16,20-22]. Nevertheless, the conclusions are still unclear and controversial. In the present study, SDF levels in the D1 samples had a significantly negative relationship with motility, which is consistent with previous reports [16,20,21]. The non-association between SDF levels and motility in the D2 and D3 samples might be attributed to the small number of samples. The association between SDF level and sperm concentration has shown conflicting results [16,22]. In our study, the SDF level showed no relationship with sperm concentration. One reason to consider is that the patients included in this study were relatively younger than in other studies.
In conclusion, we demonstrated that repeated ejaculates from patients with a variety of cancers did not show a substantial variation in SDF levels. Further large-scale studies are required to investigate the sperm quality of repeated ejaculates, including D4 and D5 samples.
Notes
Conflict of interest
Byung Chul Jee is an Editor-in-Chief and Seul Ki Kim is an Associate Editor of the journal, but they were not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Author contributions
Conceptualization: SKK, BCJ. Data curation: SKK, HP, JRL. Formal analysis: SKK, HP, BCJ. Methodology: SKK, BCJ. Project administration: all authors. Visualization: SKK, BCJ. Writing–original draft: SKK, BCJ. Writing–review & editing: all authors.
Acknowledgements
The authors thank the Division of Statistics in the Medical Research Collaborating Center at Seoul National University Bundang Hospital for their statistical analyses.