Preimplantation genetic testing for aneuploidy: The management of mosaic embryos
Article information
Abstract
As the resolution and accuracy of diagnostic techniques for preimplantation genetic testing for aneuploidy (PGT-A) are improving, more mosaic embryos are being identified. Several studies have provided evidence that mosaic embryos have reproductive potential for implantation and healthy live birth. Notably, mosaic embryos with less than 50% aneuploidy have yielded a live birth rate similar to euploid embryos. This concept has led to a major shift in current PGT-A practice, but further evidence and theoretically relevant data are required. Proper guidelines for selecting mosaic embryos suitable for transfer will reduce the number of discarded embryos and increase the chances of successful embryo transfer. We present an updated review of clinical outcomes and practice recommendations for the transfer of mosaic embryos using PGT-A.
Introduction
In the field of preimplantation genetic testing for aneuploidy (PGT-A), mosaicism was first identified 25 years ago in a validation study, where it was thought to be caused by an insufficient trophectoderm (TE) sample size [1]. Technological innovations, such as next-generation sequencing (NGS), have significantly improved the identification and quantification of mosaicism. Some authors recently proposed that an “intermediate copy number” of individual chromosomes is a more accurate term than mosaicism [2].
Mosaic embryos have the potential to implant and develop into genetically normal babies [3,4]. Greco et al. [5] first reported in 2015 that 18 women who had mosaic embryo transfers gave birth to six healthy euploid newborns. In a recent prospective study, the authors demonstrated that mosaic embryos had a similar implantation rate (55% vs. 55.8%, p=0.86) and live birth rate (43.4% vs. 42.9%, p=0.82), as well as equivalent developmental potential to that of euploid embryos [6]. In addition, multicenter studies found no significant differences between euploid and mosaic embryo transfers in terms of the preterm delivery rate, birth weight, or risk of congenital malformations [3,7,8].
However, arguments for and against transferring mosaic embryos still exist [9]. The International Do No Harm Group in in vitro fertilization (IVF) argued against the 2019 Preimplantation Genetic Diagnosis International Society (PGDIS) guideline for mosaic embryo transfer [10] on the basis that the interpretation of mosaicism in PGT-A was misleading [9,11]. On the contrary, a recently published prospective non-selection study reported that the risk of clinical error in the diagnosis of uniform aneuploidy by NGS-based PGT-A was exceedingly low (0%–2%), suggesting that PGT-A has high predictive power [12]. Given the variability in the management of mosaic embryos, it is important for clinicians to have informative genetic counseling resources available when informing their patients of PGT-A results and giving recommendations for mosaic embryo transfer. Therefore, we aimed to provide the latest clinical outcomes following mosaic embryo transfers in PGT-A cycles and a summary of updated practice recommendations.
Definition and types of mosaicism
Mosaicism is the presence of more than one genotypically distinct cell population within a single zygote [13]. Mosaic cellular populations are thought to arise from post-zygotic mitotic errors during post-zygotic cell division [13]. In PGT-A, mosaicism is defined as a mixture of 20% to 80% aneuploid and euploid DNA content; those with less than 20% aneuploid DNA are called euploid, and those with more than 80% aneuploid DNA are called aneuploid. [14]. The incidence of mosaic embryos has been reported to be 5%, but some have found rates of 20%–30% using PGT-A [15]. Mosaicism is more frequently found in cleavage-stage embryos (30%–70%) [16] compared with blastocyst-stage embryos (5%–15%) [17,18].
Mosaicism can be classified into four types based on cell lineage and the timing of mitotic errors in the blastocyst stage [19,20]. An embryo is defined as “total mosaic” when both the inner cell mass (ICM) and TE contain aneuploid and euploid cells. If the mosaic population is exclusively ICM, the embryo is defined as “ICM mosaic,” and if exclusively TE, the embryo is “TE mosaic.” Finally, if all cells in the ICM are aneuploid and all cells in the TE are euploid (or vice versa), the embryo is “ICM/TE mosaic”.
Factors contributing to the diagnosis of mosaicism
Mosaicism may not be associated with maternal age [21]. Some authors suggested a slight increase in mosaicism in younger patients compared to women over 37 years of age [22]. In cases of low-degree mosaicism and segmental aneuploidies, the incidence of mosaicism showed a negative correlation with maternal age [23,24]. Contrary to the effects of maternal age, ovarian response to stimulation was positively related to the occurrence of segmental aneuploidy. In one study, the oocyte vitrification and ovarian response showed no effect on the mosaicism rate [22].
A high proportion of mosaic embryos was found in couples with low sperm concentrations [25,26]. The prevalence of mosaic and chaotic aneuploidy in blastomeres ranges from 35% to 68% in oligozoospermic and azoospermic men [27,28]. There is a higher proportion of mosaic embryos in PGT-A cycles with male infertility compared to patients with normal sperm parameters. The highest mosaicism rates were related to the severity of male infertility [25,26].
Technical laboratory factors may affect the quality of a biopsy and thus may affect the occurrence of mosaicism within the TE. The differences in platform specificity and sensitivity, the protocols for DNA amplification, and the threshold settings established for interpretation can lead to differences in the proportion of mosaicism and the number of euploid embryos to transfer [29]. Other factors associated with the biopsy technique, including the conditions surrounding cell loading and the number of cells biopsied, can also affect the results [30]. The method of fertilization [31] and laboratory conditions, such as oxygen concentration, pH and osmolality in the embryo culture medium, and temperature are related to an increased rate of mosaicism [30].
Management: transfer of mosaic embryos
Multiple factors determine the fate and viability of mosaic embryos, such as the degree of mosaicism in the biopsied sample, the specific type and number of chromosomes involved, and the type of mosaicism.
1. Priority for mosaic embryo transfer
In 2016, the position statement of the PGDIS recommended priorities for mosaic embryo transfers based on the specific chromosome involved and the level of mosaicism [32]. In 2017, the World Congress on Controversies in Preconception, Preimplantation and Prenatal Genetic Diagnosis highlighted the need for PGT-A in IVF practice and updated the PGDIS position statement on recommendations for clinical practice [33]. In 2018, Grati et al. [34] published a study on the chorionic villi samples (CVS) and products of conception (POC) after natural pregnancy to provide a practice guideline whereby mosaic embryos could lead to healthy live births. In 2020, Munne et al. [35] suggested classifying mosaic embryos into high- (>50%) and low-level (<50%) groups, with preference for transferring single segmental mosaic embryos over other types of mosaicism. In 2021, Viotti et al. [4] formulated a ranking system using outcome data from one thousand mosaic embryo transfers for the prioritization of mosaic embryos in the clinical setting. They confirmed that combined mosaic embryos have significantly lower implantation and pregnancy rates than euploid embryos. They also found that the type and level of mosaicism had a significant impact on the embryo transfer outcomes. Their study helped to elucidate the problems presented by mosaic transfer and attempted to provide firm conclusions. Relevant medical society practice guidelines and recommendations, including the recent PGDIS 2021 guidelines [36], are summarized in Table 1.
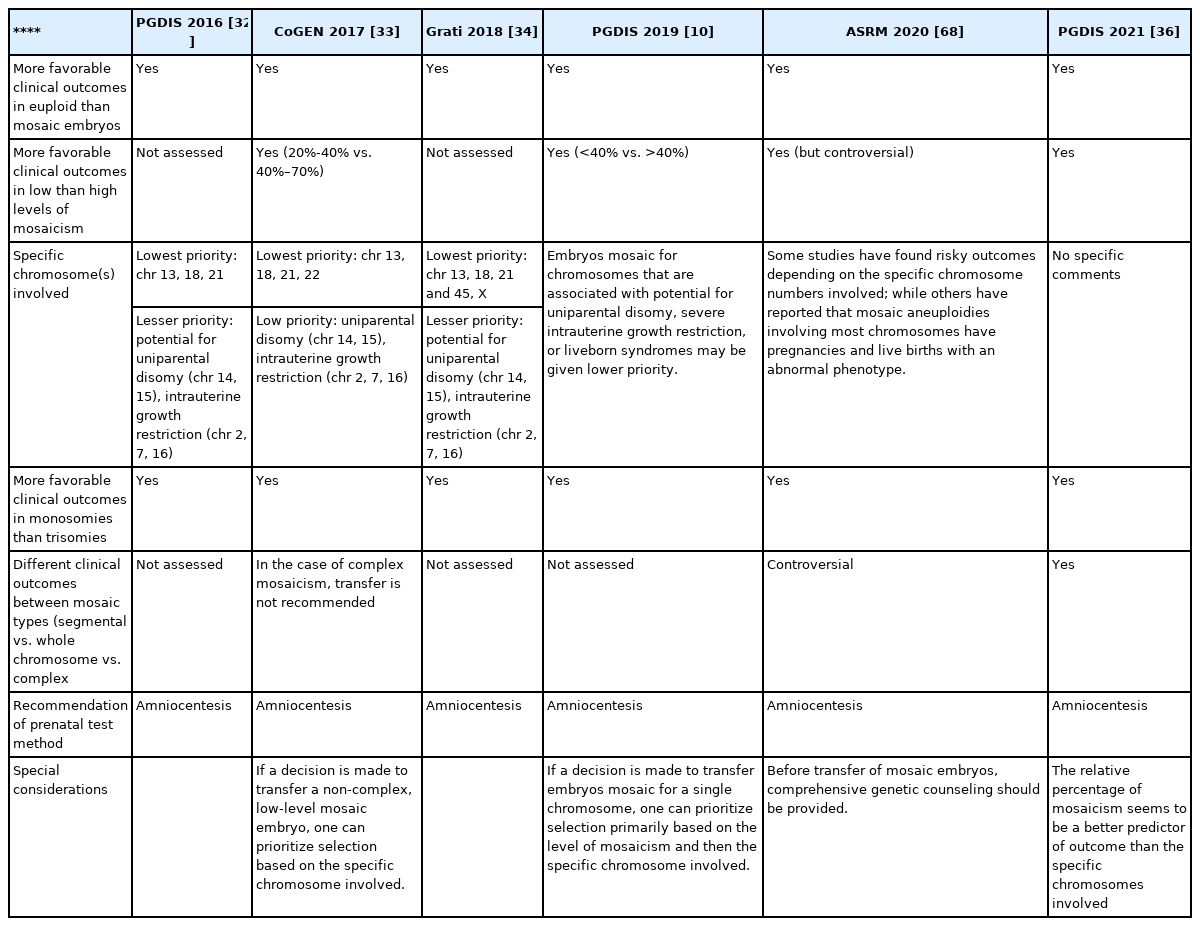
A list of professional medical society guidelines and recommendations regarding mosaic embryo transfer
Despite these diverse ranking approaches, attempts to provide clinical recommendations for patients may yet be in early stages. Uncertainty remains regarding related factors affecting the clinical outcome data of mosaic embryo transfer. Some studies have suggested differences in live-birth rates based on the type and level of mosaicism [38] or involvement of a full versus partial chromosome [39], while others have failed to find such significance using the same classification system [40].
2. The degree of mosaicism
Chromosomal mosaicism has been defined as low-level mosaicism if abnormal cells are in the 30%–50% range and high-level mosaicism if abnormal cells are in the 50%–70% range using the NGS validation algorithm [41]. Clinical outcome data related to high- versus low-level mosaicism still show conflicting results. Some studies found that low-level mosaicism was related to improvement in ongoing pregnancy rates [38], while others did not find statistically significant results [40,42,43]. Embryos with low-level mosaicism are more likely to develop into healthy babies than high-level mosaic embryos, whereas high-level mosaic embryos increase the risk of miscarriage [35,38,41,44]. A recent prospective study found that embryos with more than 50% mosaicism have a significantly lower implantation rate (24.4% vs. 54.6%; p<0.002), clinical pregnancy rate (15.2% vs. 46.4%; p<0.001), and live birth rate (15.2% vs. 46.6%; p<0.001) compared to euploid embryos in the NGS profile [38]. Capalbo et al. [6] showed that low- (20%–30%) or moderate-degree (30%–50%) mosaic embryo transfer yielded similar clinical and neonatal outcomes in a prospective double-blinded non-selection trial.
3. Specific chromosomes involved
The clinical outcomes of mosaicism can be highly dependent on the chromosomes involved. Autosomes were ranked in order of their risk of placental insufficiency, intrauterine growth restriction, and uniparental disomy (UPD). The mosaic trisomy 16 chromosome is commonly affected in preimplantation embryos and leads to a high risk of abnormal perinatal outcomes, such as intrauterine growth restriction, preterm birth, and hypertensive disorders [45]. Chromosomes X, 21, and 22 have been reported to be susceptible to whole chromosome errors [46-48]. Chromosomes 2, 6, 7, 11, 14, 15, 16, and 20 are known to be associated with UPD [49,50]. Recent findings showed that chromosome length had a positive correlation with the mitotic error of each chromosome, but a negative correlation with the meiotic error of the preimplantation embryo [47,51].
Grati et al. [34] devised a scoring system for prioritizing mosaic embryo transfers based on the mosaic patterns observed in prenatal samples and products of conception and on the involvement of specific chromosomes. Mosaic trisomies 1, 3, 10, 12, and 19 had top priority for embryo transfer because of their low risk of deleterious outcomes, whereas mosaic trisomies 13, 16, 18, 21, 45, and monosomy X had a high risk of nonviable births and should be avoided. However, this system was limited by the difficulty found in assessing the degree of mosaicism in preimplantation embryos based only on molecular and cytogenetic results.
4. Monosomies versus trisomies
Most monosomies arise from mitotic errors and most trisomies result from nondisjunction during maternal meiotic errors [17,52]. Since monosomic cells are less likely to be viable than trisomic cells [53], most monosomic cells are removed at the post-implantation phase [54,55]. Trisomic mosaicism can occur in live births with chromosomal aneuploidy and is associated with cognitive and physical impairments [56]. Although PGDIS recommended the transfer of embryos with mosaic monosomies over those with mosaic trisomies in 2016 [32], this statement was updated and removed in 2019 [10]. In addition, some authors did not find a significant difference in pregnancy rates between monosomic and trisomic mosaic embryos [40]. According to the PGDIS guidelines, mosaic trisomies 1, 3, 4, 5, 6, 8, 9, 10, 11, 12, 17, 19, 20, 22, X, and Y are preferred over mosaic trisomies 2, 7, 13, 14, 15, 16, 18, and 21 when mosaic trisomy is being considered for transfer [32]. Recent studies proposed that mosaic monosomies and mosaic trisomies have similar implantation rates (46% and 47.2%, respectively; p>0.05) and ongoing pregnancy rates (36% and 33%, respectively; p>0.05) [4,33].
5. Whole versus segmental aberrations
When duplication or deletion errors occur in a small portion of DNA during mitotic division, the embryo will have a mosaic of the segmental error, allowing some cells to have a normal copy number of chromosomes and others to have segmental deletion or duplication of the chromosomes [57]. In one study, segmental gain or loss was affected in 25% of mosaicism [58]. Some authors suggested that the incidence of segmental mosaicism may be overestimated due to biological and technical errors [59]. Clinical perspectives of embryo mosaicism, with respect to full versus partial aneuploidies, have been inconsistent. Some studies have reported a higher clinical pregnancy rate in partial aneuploid mosaicism [39,42,60], while others have not found a significant difference [40]. In the subgroup of segmental mosaic embryos, Viotti et al. [4] recently investigated clinical outcomes after the transfer of 1,000 mosaic embryos and reported similar implantation rates (51.6% vs. 57.2%; p=0.001) and ongoing pregnancy rates (43.1% vs. 52.3%; p=0.001) compared to euploid embryos. Other recent studies also revealed that segmental mosaic embryos had clinical outcomes comparable to euploid embryos [12,61].
Regarding chromosome type, large chromosomes such as chromosomes 1 to 9 are prone to breakage, resulting in segmental mosaicism [62,63], while a significantly lower percentage of copy number errors were observed in small chromosomes and acrocentric chromosomes (e.g., chromosomes 19, 21, 22, and Y) [64,65].
Segmental aneuploidies originate because of mitotic errors during preimplantation development [24]. This is related to blastocyst morphology, not to maternal age or clinical and embryological parameters [66]. A previous multicenter study of 822 mosaic embryo transfers demonstrated that the reproductive potential of mosaic embryos is affected by the number of euploid cells and the complexity in the TE biopsy sample [67]. The embryos with segmental aneuploidy had better clinical outcomes than mosaic embryos with one or two involved chromosomes (implantation rate: p<0.001, ongoing pregnancy rate/birth rate: p<0.001).
6. The number of chromosomes involved (single versus double versus complex aneuploidies)
Several studies found reduced pregnancy capacity in mosaic embryos that had three or more chromosomes involved [40] and in segmental mosaicism that had two or more chromosomes involved [42], whereas other studies did not report clinically significant differences between mosaic embryos involving one or two chromosomes [40,68]. Complex mosaic embryos had the lowest implantation rates among single aneuploid, double aneuploid, and segmental mosaic embryos [40].
Genetic counseling
A recent statement by the American Society for Reproductive Medicine highlighted the importance of patient education prior to PGT-A [37]. Before the transfer of mosaic embryos, counseling should include a discussion of the potential challenges in interpreting mosaic results, the potential risks of mosaic embryo transfers, and the limited neonatal outcome data available. In addition, counseling should provide information regarding the genetic advantages, risks, and limitations of a prenatal diagnosis. Thus far, most prenatal testing results after mosaic embryo transfers have shown normal healthy fetuses with no specific chromosomal abnormalities [3]. However, we found two reports of babies with abnormal karyotypes: a baby with 15q duplication syndrome after transfer of a 57% segmental mosaic embryo [69] and a healthy baby with 2% mosaic monosomy 2 after transfer of a 35% mosaic monosomy 2 embryo [70].
Patients should be informed about the risk of mosaicism in a biopsy specimen, the complexities of the various possible outcomes after transfer of a mosaic embryo, and the need for close prenatal monitoring, including amniocentesis. Until definitive data is available, patients should be advised to go through additional cycles if possible to obtain euploid embryos instead of transferring a mosaic embryo. A schematic prioritization of mosaic embryos according to clinical outcomes is shown in Table 2.
Prenatal diagnosis after transfer of mosaic embryos
If a pregnancy has been confirmed after mosaic embryo transfer, prenatal diagnosis is recommended to identify fetal chromosomes and other genetic conditions. Although evidence-based guidance for prenatal testing after mosaic embryo transfer is still lacking, most practice statements consistently recommend amniocentesis as the gold standard for prenatal diagnosis [32,33,37,71]. Karyotyping of the amniocytes obtained by amniocentesis is done to diagnose aneuploidy in the fetus [72]. CVS can be useful for patients seeking a diagnosis during the first trimester; however, CVS results represent placental cells derived from the TE. Thus, mosaic findings detected using CVS may indicate placental mosaicism, and follow-up amniocentesis is required to clarify the results. The major advantage of amniocentesis is the ability to analyze fetal cells directly, but it may miss low-level mosaicism. Therefore, amniocentesis best represents the chromosome complement within fetal tissues, but patients should know that some mosaicism may not be detectable. Depending on the PGT-A result, further analysis of prenatal samples should also be considered; chromosomal microarray can be performed if segmental aneuploidy or UPD is involved [37,73]. Cell-free DNA (cfDNA) testing, also known as noninvasive prenatal testing (NIPT), has not been validated to detect mosaicism because NIPT analyzes circulating cfDNA fragments in the maternal plasma derived from both the mother’s and apoptotic trophoblasts, but not from the fetus itself [74].
Conclusion
Although interest in mosaic embryo transfers is increasing, the debate over whether mosaic embryos can be transferred is ongoing. In practice, the identification of mosaic subgroups that are viable and worthy of transfer is very important, but it is also vital to inform patients that the data on postnatal and neonatal outcomes following mosaic embryo transfers are still limited and that clinical outcomes have been mixed. We emphasize the need for further research on the genetic and clinical outcomes of mosaic embryo transfers. Large-scale multicenter studies would be of particular value in collecting data for the risk evaluation of mosaic embryo transfers and could potentially reduce the disposal of viable embryos for implantation and live births.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Author contributions
Conceptualization: ISK. Writing–original draft: EJY. Writing–review & editing: all authors.

