The impact of hyperandrogenism on the outcomes of ovulation induction using gonadotropin and intrauterine insemination in women with polycystic ovary syndrome
Article information
Abstract
Objective
This study aimed to investigate the impact of hyperandrogenism (HA) on the outcomes of ovulation induction (OI) using gonadotropin and intrauterine insemination (IUI) in patients with polycystic ovary syndrome (PCOS).
Methods
This was a retrospective cohort study including 415 patients undergoing OI using gonadotropin and IUI treatment between January 2018 and December 2020 at a single infertility center. Baseline characteristics, clinical and laboratory parameters, and pregnancy outcomes were investigated.
Results
Among the study population, there were 105 hyperandrogenic (25.3%) and 310 non-hyperandrogenic patients (74.7%). The live birth rate was lower in the HA group than in the non-HA group, but this difference did not reach statistical significance due to the limited sample size (14.3% vs. 21.0%, relative risk=0.68; 95% CI, 0.41–1.14, p=0.153). No predictive factors for live birth were identified through logistic regression analysis.
Conclusion
HA did not negatively affect the outcomes of OI using gonadotropin and IUI cycles in Vietnamese women with PCOS. The result may not be applicable elsewhere due to the large variation in the characteristics of women with PCOS across races and populations.
Introduction
Polycystic ovary syndrome (PCOS) is a common neuroendocrine disorder, affecting 6%–9% of women of reproductive age [1]. According to the Rotterdam consensus (2003), the diagnosis of PCOS is based on the presence of at least two out of three groups of symptoms: ovulatory dysfunction, hyperandrogenism (HA), and polycystic ovary morphology (PCOM) on ultrasonography [2]. The health problems associated with this syndrome are diverse and have significant negative impacts on quality of life and fertility [3].
For sub-fertile women with PCOS, lifestyle modifications, such as regular physical activity, healthy eating habits, and diet balancing, are the first-line treatment options for infertility [4-6]. When lifestyle modifications fail, ovulation induction (OI) is a simple, non-invasive, low-cost approach that can be considered an alternative [6]. The most common drug of choice is clomiphene citrate and gonadotropin, while letrozole and metformin may also be used off-label [6]. OI with intrauterine insemination (IUI; OI+IUI) is the option of choice when there is coexisting suboptimal semen quality [7]. Although the effectiveness is unclear, performing IUI in ovulation-induced cycles is widely used for women with PCOS without male-related factors [8].
In women with PCOS, it is postulated that HA plays a critical role in the origins of PCOS [9]. Studies have found that HA remodels follicular development competence [10] and increases the risk of miscarriage and other adverse maternal-fetal outcomes, especially in Asian women [11,12]. In addition, HA has been demonstrated to increase hypertension in pregnancy, leading to preterm birth [13]. HA has been found to negatively affect the live birth rate (LBR) in women with PCOS after assisted reproductive techniques [14]. However, there have not been many studies on the effects of HA on OI and the outcomes of IUI. Thus, we decided to perform this study to evaluate the impact of HA on the treatment outcomes of OI using gonadotropin and IUI.
Methods
1. Study setting and population
This was a retrospective study at IVFMD, My Duc Hospital, Ho Chi Minh City, Viet Nam analyzing women with PCOS between January 2018 to December 2020. The study was approved by the Institutional Review Boardof My Duc Hospital (08/21/DD-BVMD), on August 3, 2021. Patients’ information was kept confidential. All treatment data were agreed to be used for scientific research purposes.
Infertile women with PCOS aged 18 to 38 years who underwent the first cycle of OI with gonadotropin followed by IUI were eligible for the study. Patients were diagnosed with PCOS based on the Rotterdam criteria and must have had at least one patent Fallopian tube, as shown on hysterosalpingography. In addition, the male partner had normal sperm or mild male factor infertility (total sperm count ≥10 million). Women with uterine abnormalities (submucosal fibroids, intra-uterine cavity polyps, bicornuate uterus, and synechiae of the uterine cavity), tubal damage, male factor infertility, severe male factor infertility, or using frozen semen were excluded.
Based on the Rotterdam criteria, patients were diagnosed with PCOS when they met at least two of the following criteria: HA (modified Ferriman-Gallway score ≥3 [6,15], a total testosterone level ≥1.8 nmol/L [16], or a free androgen index >6 [17]); ovulation dysfunction (cycle length <21 or >35 days or <8 cycles/year or amenorrhea (>90 days); PCOM (≥20 follicles per ovary or ovarian volume of >10 mL on transvaginal ultrasonography using transducers with a frequency bandwidth that includes 8 MHz, ensuring no corpora lutea, cysts, or dominant follicles were present). There were two groups of patients in this study: hyperandrogenic (HA) and non-hyperandrogenic (non-HA) women.
2. IUI procedure
From day 2 to day 4 of the menstrual cycle, OI was performed using human menopausal gonadotropin (hMG; IVF-M 75 IU, LG Chem, Seoul, Korea). The administered daily dose of hMG was 75 IU/day. Doses were individually adjusted based on the ovarian response, with a maximum daily dose of 150 IU. Monitoring was performed according to the clinic’s procedures. Transvaginal ultrasonography was performed using transducers with a frequency bandwidth of 8 MHz (Samsung HS30, Seoul, Korea) to measure follicles’ diameters. Patients were scheduled for a check-up on day 7 of stimulation. After that, follicular monitoring was performed every 2–3 days, depending on the number and size of follicles. Ovulation was triggered when the leading follicle’s diameter reached 18 mm, using human chorionic gonadotropin (IVF-C 5000 IU, LG Chem) at a dose of 5,000 IU. The IUI cycles were canceled or converted to in vitro fertilization (IVF) or in vitro maturation (IVM) for patients who had (1) more than three follicles with a diameter of ≥14 mm observed or (2) ovarian unresponsiveness to the hMG maximum daily dosage of 150 IU after 21 days of stimulation. In patients who had three or more follicles with a diameter of 14 mm but refused to cancel IUI cycle, a bolus of gonadotropin-releasing hormone (GnRH) agonist (Diphereline 0.1 mg; Ipsen Pharma Biotech, Signes, France) at a dose of 0.1 mg was indicated to induce ovulation.
IUI was performed around 36 to 40 hours after ovulation triggering. The couples were instructed to have regular intercourse during stimulation, with the last intercourse to be no more than 2 days prior to insemination. Semen was collected and washed within 1 hour using both the swim-up technique and sperm density gradient centrifugation. The volume of the prepared semen sample used for insemination was 0.4 mL. Insemination was subsequently performed by physicians using a soft catheter (Gynétics, Lommel, Belgium). Bed rest after IUI was optional, depending on patients’ preferences.
Micronized progesterone (Cyclogest 200 mg; 400 mg/day, vaginal; Actavis, Parsippany-Troy Hills, NJ, USA) was used for luteal phase support for 14 days after insemination. A pregnancy test was performed by measuring the serum beta human chorionic gonadotropin (β-hCG) level 2 weeks after IUI. A level of β-hCG of 5 mIU/mL or above was considered pregnancy. Transvaginal ultrasonography was performed 3 weeks later.
3. Outcome measures
The primary outcome was the LBR. Live birth was defined as an infant born after 24 weeks with vital signs, heart rate, and muscle tone [18]. The secondary outcomes were the positive β-hCG, clinical pregnancy, ongoing pregnancy, ectopic pregnancy, miscarriage rates; the multiple pregnancy rate; the rates of ovarian hyperstimulation syndrome (OHSS); hypertensive disorders of pregnancy (HDP), and gestational diabetes mellitus (GDM); the rate of cycles with mono-/multi-follicular growth; and the rates of cycle cancellation and cycles converted to IVF or IVM.
4. Statistical analysis
Data were analyzed using descriptive statistics (mean and standard deviation for normally distributed variables, or median and interquartile range for skewed variables). Differences between groups were analyzed using one-way analysis of variance with the post hoc Tukey honest significant difference test or the Kruskal Wallis test for normally distributed or skewed variables, respectively, and the chi-square test for categorical variables. Univariable and multivariable logistic regression analyses were performed to identify factors associated with live birth. All variables with a p-value <0.25 in the univariate analysis were included in the multivariable analysis. All analyses were performed using the R statistical package (R version 3.3.3; R Foundation, Vienna, Austria). Statistical significance was defined as p<0.05.
Results
1. Baseline characteristics
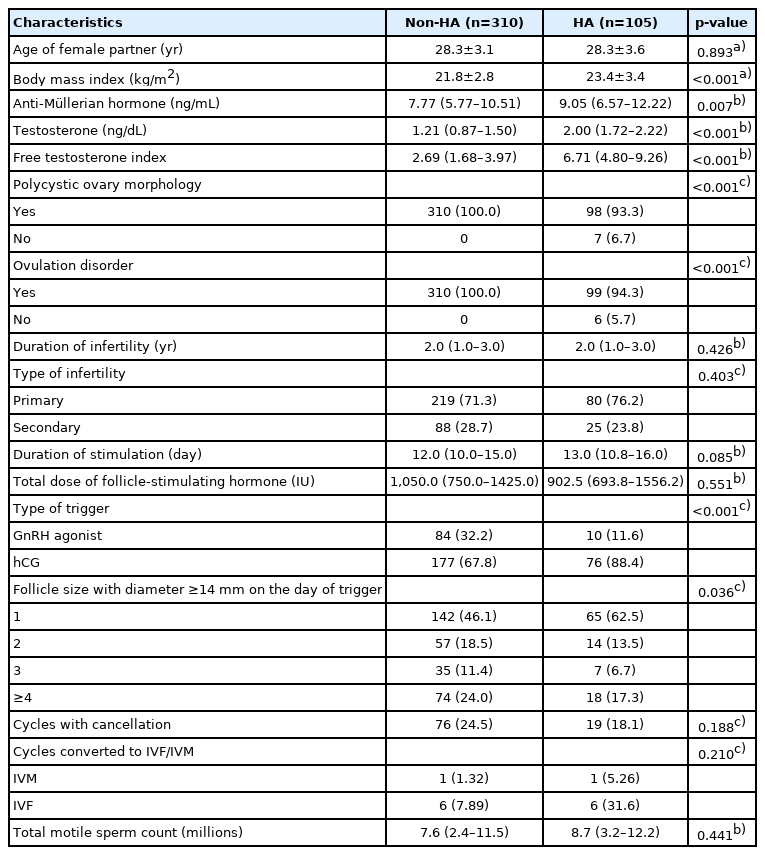
In total, 415 women with PCOS were enrolled in this study from January 2018 to December 2020. Of these patients, 105 (25.3%) were diagnosed with HA, and 310 did not have HA (74.7%). The women in this study were relatively young, with a mean age of 28.3 years. Both the HA and non-HA women were non-obese, with a mean body mass index (BMI) of 23.4 and 21.8 kg/m2, respectively. The anti-Müllerian hormone level was significantly higher in the HA women than in the non-HA women (9.05 vs. 7.77 ng/mL, respectively). Most non-HA women had PCOM, while the prevalence was 93.3% in HA women. In HA group, there were seven cases without PCOM and six cases without ovulation dysfunction. The types and duration of infertility and the total motile sperm count were comparable between the two groups defined according to the PCOS phenotype. The total gonadotropin consumption and the duration of ovarian stimulation did not differ between the two groups. In most cycles, there was only one dominant follicle, and the most common method for ovulation triggering was hCG. There was also no difference in the cancellation rate. Similarly, the IVM and IVF conversion rates were comparable. Patients’ demographic and clinical characteristics are shown in Table 1.
2. Treatment outcomes
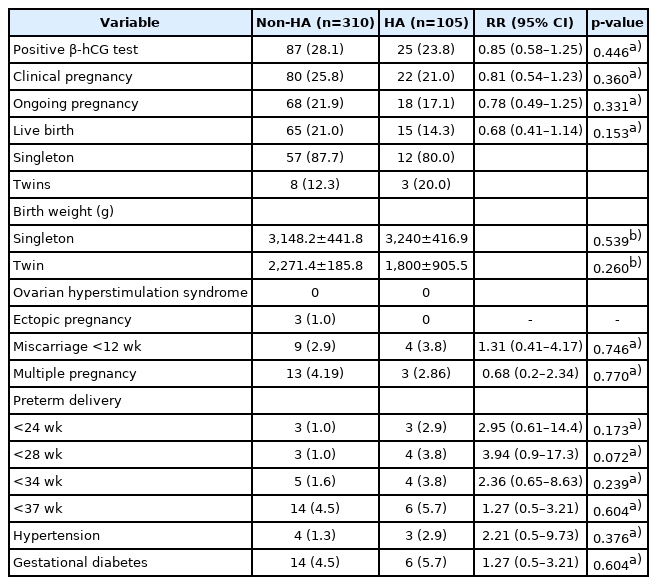
Overall, the LBR in HA women was lower than in non-HA women (14.3% and 21.0%, respectively). However, statistical significance was not reached (p=0.153). The majority of pregnancies resulted in singletons. The prevalence of pregnancies with twins in HA and non-HA women was 20% and 12.3%, respectively. The birth weights of babies born were also comparable between the two groups. There were no significant differences between the two groups regarding the rates of positive pregnancy tests, ectopic pregnancies, miscarriages, and preterm births. The percentages of pregnancies with HDP and GDM were comparable between the two groups. There were no cases of OHSS. The details related to treatment outcomes are shown in Table 2.
No predictive factors for live birth were identified after logistic regression analysis (Table 3). There was no correlation between obesity and the treatment outcomes in women with HA (Supplementary Table 1).
Discussion
Our study evaluated the impact of HA on OI+IUI outcomes and reported long-term treatment outcomes. The results from our study demonstrated that the LBR was lower, although not significantly, in the HA group than in the non-HA group of women undergoing OI+IUI treatment due to the limited sample size. Our study has certain limitations. The first limitation is the retrospective nature of the study. Secondly, this is a single-center study that may not fully represent the overall population of women with PCOS. Thirdly, the study was performed among Vietnamese women, which may limit the generalizability of the findings due to the differences in characteristics of women with PCOS across races and populations.
The results from our study showed an LBR consistent with those reported by previous studies investigating IUI outcomes in women with PCOS. Huang et al. [19] conducted a study on 1068 IUI cycles and reported an overall LBR of 13.2%. In cycles with multi-follicular growth, the LBR was slightly higher, at 15.8%. It is also worth noting that 49.9% of cycles in our study achieved mono-follicular growth. The LBR in our study was also comparable to the LBR in cycles included in a systematic review [20]. The percentages of cancellation or conversion to IVF and IVM treatment were comparable between HA and non-HA groups. Previous studies on IUI considered factors such as age [21], obesity [22], ovulation dysfunction [23,24], ovarian reserve [21,25-27], and the presence of HA [28] as predictors for pregnancy. This study could not demonstrate the hypothesis that HA has a negative impact on pregnancy outcomes after OI+IUI. This contrasts with results from the latest systematic review by Ma et al. [11], which stated that the rates of clinical pregnancy, miscarriage, and adverse pregnancy outcomes were higher in patients with HA. Moreover, De Vos et al. [29] found that the c (CLBR) after fresh or frozen embryo transfer in patients with hyperandrogenic PCOS phenotypes was significantly lower than in normoandrogenic patients. In particular, the CLBR of the hyperandrogenic phenotypes A and C were 25.8% and 27.8%, compared with the rates of 48% in patients with the normoandrogenic phenotype D (p=0.002 and p=0.01, respectively) and 53.3% in controls with polycystic ovarian morphology (p<0.001 and p=0.001, respectively) [29]. The median free testosterone index of patients in our study was low, at a level of 6.71 (Q1=4.80, Q3=9.26). This is similar to the findings of another study in Vietnamese women with PCOS by Cao et al. [30]. Given the fact that the free testosterone index in our study was impressively lower than that of other ethnicities [11,28], it was hypothesized that the severity of HA in our patients was less than that of different populations. Moreover, the presence of HA can likewise potentially affect treatment outcomes differently in Vietnamese individuals. There is an essential role of obesity in treatment outcomes in women with PCOS in the interaction with HA. For patients undergoing IVF or intracytoplasmic sperm injection, Romanski et al. [31] showed a significant trend for a decreased LBR and increased miscarriage rate as BMI increased. Furthermore, patients with a BMI >40 kg/m2 had worse IVF treatment outcomes than normal-weight patients. High BMI could also affect OI+IUI treatment outcomes negatively. A recent retrospective study by Guan et al. [22] investigating 831 IUI cycles showed that obese women might require more gonadotropin doses and more days of stimulation. Moreover, obesity is recognized in the literature as an aggravating factor of endocrine-metabolic disorders, insulin resistance, response to ovarian stimulation, and adverse events in pregnancy and the neonatal period [32-38]. As mentioned previously, the women in our study were non-obese. This is similar to other studies showing a lower prevalence of obesity in East Asian women with PCOS than in other populations such as Hispanic, Caucasian, and African descent [39-42]. Therefore, the low BMI could explain the consistency in treatment outcomes between both groups of patients in our study. In other words, in our less severely hyperandrogenic and non-obese patients, the effects of the PCOS phenotype on treatment outcomes may not differ. Additionally, a subgroup analysis was also performed in order to further investigate the impact of obesity on treatment outcomes in HA women. Similarly, there was no significant difference between non-obese and overweight or obese HA women.
There are still many concerns about gonadotropin administration in OI because of the high occurrence of OHSS and multiple pregnancies associated with its use. There were no cases of OHSS in our study. A possible reason could be the strict implementation of an OHSS prevention strategy at our center, including a GnRH agonist trigger. A GnRH agonist trigger was indicated when there were more than three follicles at a diameter of ≥14 mm on the day of trigger. The percentage of cycles with a GnRH agonist trigger was significantly lower in the HA group than in the non-HA group (11.6% vs. 32.2%, p=0.01). However, there was no significant difference in the multiple pregnancy rate between the two groups (2.86% vs. 4.19%, p=0.77). The percentage of twins was also comparable, and no higher-order multiple pregnancies were recorded. This incidence was similar to that of the aforementioned study [15].
In conclusion, HA in Vietnamese women with PCOS did not have a negative effect on OI+IUI outcomes, unlike the findings of previous studies in other races. The result may not be applicable elsewhere due to the large variation in the characteristics of women with PCOS across races and populations.
Supplementary material
Supplementary material can be found via https://doi.org/10.5653/cerm.2022.05204.
The correlation between different BMI levels and treatment outcomes in HA women
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Author contributions
Conceptualization: VNAH, TDP, NTN, TMH, LNV. Data curation: VNAH, TDP, LNV. Formal analysis: VNAH, TDP, NTN, LNV. Methodology: VNAH, TDP, TMH, LNV. Project administration: VNAH, TDP, TMH, LNV. Visualization: VNAH, TDP, NTN, HLTH, LNV. Writing–original draft: VNAH, TDP, NTN, HLTH, LNV. Writing–review & editing: all authors.