Impact of imatinib administration on the mouse ovarian follicle count and levels of intra-ovarian proteins related to follicular quality
Article information
Abstract
Objective
The impact of imatinib, a tyrosine kinase inhibitor, on ovarian follicles and several proteins related to follicular function and apoptosis was investigated in mice.
Methods
Saline, cyclophosphamide (Cp; 50 or 75 mg/kg), or imatinib (7.5 or 15 mg/kg) was injected once intraperitoneally into female B6D2F1 mice (18 mice in each group). In multiple ovarian sections, the number of various types of follicles and the proportion of good-quality (G1) follicles were counted. The levels of six proteins (anti-Müllerian hormone [AMH], BCL-xL, BAX, acid sphingomyelinase [A-SMase], caspase-3, and α-smooth muscle actin [α-SMA]) within the whole ovaries were quantified using Western blots.
Results
Compared to the saline group, a significant reduction of the primordial follicle count was observed in the group treated with imatinib 7.5 and 15 mg/kg, as well as in the group treated with Cp 75 mg/kg. Administration of Cp significantly decreased the proportion of G1 primordial follicles, but administration of imatinib did not. No differences in the AMH, anti-apoptotic BCLX-L, pro-apoptotic BAX, and A-SMase levels in the ovarian tissues were observed among the five groups. However, caspase-3 and α-SMA levels were significantly higher in the imatinib and Cp groups than in the saline group.
Conclusion
The administration of imatinib to mice significantly reduced the primordial follicle count and increased the protein levels of caspase-3 and α-SMA. Our findings suggest that imatinib potentially exerts ovarian toxicity via apoptotic processes, similarly to Cp.
Introduction
Imatinib, a tyrosine kinase inhibitor, is widely used in patients with chronic myeloid leukemia (CML) or gastrointestinal stromal tumors [1,2]. Although the detrimental effects of chemotherapeutics on future fertility are a major concern for female cancer survivors, it is largely unknown whether imatinib causes ovarian damage [3].
The main target of imatinib is an oncogenic protein formed by the BCR-ABL fusion gene. Imatinib also inhibits other tyrosine kinases such as ABL, KIT, and platelet-derived growth factor receptor (PDGFR) [4-6]. Within the ovary, the KIT ligand and PDGF have been shown to independently promote primordial follicle activation, transition from the primordial to primary follicle, oocyte growth, granulosa cell proliferation, and follicle survival [7-9]. Because imatinib inhibits the c-KIT pathway, which is essential for ovarian follicle development, it may have a negative impact on ovarian follicle survival [10].
To date, studies on ovotoxicity of imatinib in humans are scarce. A case series reported that amenorrhea was usually not induced in women taking imatinib orally, and successful conception commonly occurred [11]. However, a few case reports have suggested that imatinib may induce the loss of ovarian reserve. One case report showed that long-term administration of imatinib (for 2 years) might lead to primary ovarian insufficiency [12]. In another case report, an imatinib user showed a severely impaired ovarian response to exogenous gonadotropin stimulation, but presented a normal ovarian response after stopping imatinib [13].
Several animal experiments have reported unclear results regarding the potential ovotoxic effects of imatinib. In a zebrafish model, imatinib feeding once, twice, or three times per day caused frequency-dependent irreversible suppression of ovarian folliculogenesis [14]. In a mouse model, long-term injections of imatinib (for 4–6 weeks) induced diminished ovarian reserve [15]. In that report, mice treated with imatinib could yield in vivo fertilized zygotes through ovarian stimulation, but the development of zygotes in vitro and implantation of subsequent blastocysts were severely hampered [15].
Intraperitoneal injections of imatinib in human ovary-xenografted mice increased follicular atresia and induced bizarre-shaped follicles without oocytes [16]. Meanwhile, in a leukemic mouse model, administration of imatinib orally for two months did not affect the numbers of primordial, primary, and secondary follicles [17].
To our knowledge, the effect of imatinib on ovarian function is still unclear. Thus, we aimed to investigate the effect of one-time imatinib injections in mice on the quantity and quality of ovarian follicles and the levels of six proteins: anti-Müllerian hormone (AMH), BCL-xL, BAX, acid sphingomyelinase (A-SMase), caspase-3, and α-smooth muscle actin (α-SMA). Cyclophosphamide (Cp), an alkylating drug used in cancer treatment protocols, is a well-known ovotoxic agent that induces follicle loss. We also tested the deleterious effects of Cp injections on ovarian follicles and compared the results to those of imatinib injections.
Methods
1. Mice
Six- to seven-week-old B6D2F1 female mice (Orient Bio, Seongnam, Korea) were used. They were raised in controlled sterile conditions at 22°C with a 12-hour light/dark cycle, and had free access to autoclaved pellet diet and water. The experimental protocols and animal handling procedures were ethically performed with the approval of the Institutional Animal Care and Use Committee (IACUC) of the Seoul National University Bundang Hospital (IACUC No. 53-2019-014).
2. Experimental design
After 1 week of adaptation, 90 mice were divided into five groups, and each agent was injected intraperitoneally once: 0.1 mL of normal saline (control group), Cp (Cp monohydrate; Sigma Aldrich, St Louis, MO, USA) 50 mg/kg, Cp 75 mg/kg, imatinib (Enzo Life Sciences, Farmingdale, NY, USA) 7.5 mg/kg, or imatinib 15 mg/kg. Cp was dissolved in phosphate-buffered saline (PBS) and prepared at different concentrations. The dose of imatinib was based on equivalence to the average human oral dose of 400–800 mg/day to treat CML (corresponding to 8–16 mg/kg in a human of 50 kg) [14,18]. A one-time injection of imatinib 7.5 mg/kg was also used in a previous mouse model study [19], whereas in this study, we used two doses of imatinib (7.5 mg/kg and 15 mg/kg). Imatinib powder was dissolved in PBS to obtain an imatinib stock solution. The final volume of intraperitoneal injection was all set at 0.1 mL. One week later, we sacrificed the mice by cervical dislocation and collected the bilateral ovaries.
3. Histological examinations and follicle counts
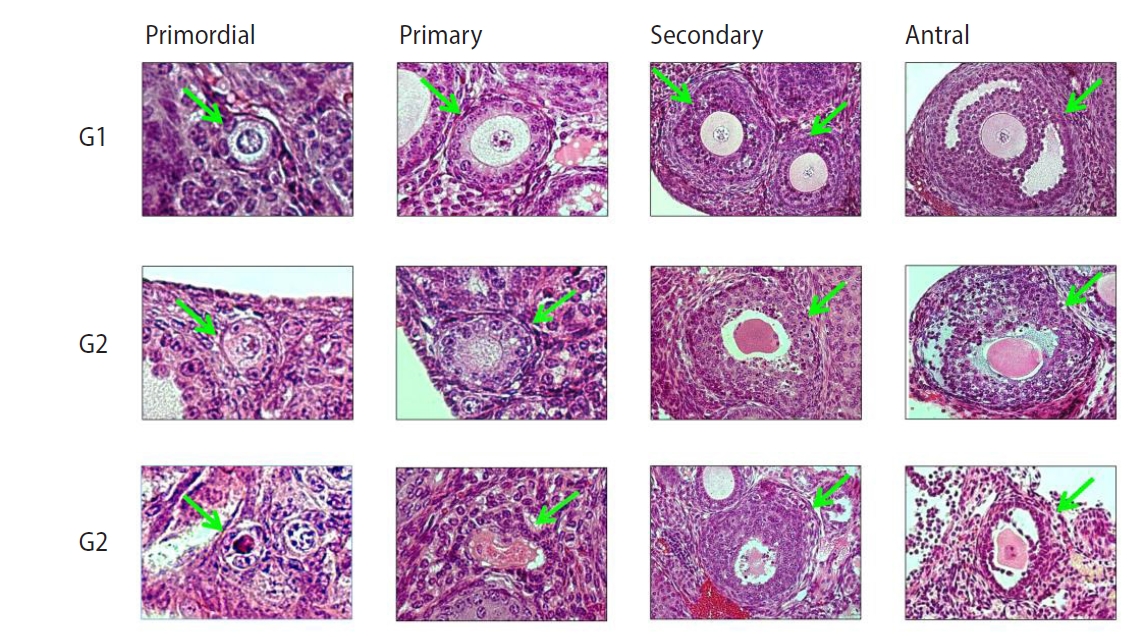
Nine mice in each group were used; thus, 18 ovaries in each group were obtained. All ovaries were fixed in 4% buffered paraformaldehyde, embedded in a paraffin block, and then cut into 4-µm sections serially, resulting in at least five sections per ovary. The ovarian sections were stained with Mayer’s hematoxylin-eosin solution (Merck-Serono, Darmstadt, Germany) for histologic examinations. Ovarian follicles were evaluated by two senior experts. Under a light microscope (Nikon, Tokyo, Japan) at ×400 magnification, ovarian follicles were classified into four types as defined in a previous study [20]: (1) Primordial follicle: a single layer of flattened pre-granulosa cells; (2) Primary follicle: a single layer of granulosa cells, including cuboidal forms; (3) Secondary follicle: at least two layers of cuboidal granulosa cells; (4) Antral follicle: multiple layers of cuboidal granulosa cells with an antrum.
Each follicle was evaluated for its integrity according to the following criteria as mentioned in a previous study [21]: (1) G1 (good quality) follicle: intact spherical follicle and oocyte; (2) G2 (fair quality) follicle: granulosa cells pulled away from the edge of follicles, but with an intact oocyte; (3) G3 (poor quality) follicle: disruption and/or loss of granulosa-theca cells, with pyknotic nuclei and/or a missing oocyte. Representative histological images of ovarian follicles are shown in Figure 1.
4. Western blotting
Nine other mice in each group were used for the Western blot analysis. The ovaries were suspended in a lysis buffer (20 mM Tris-HCl at a pH of 8.0, 137 mM NaCl, 1% Nonidet P-40, and 10% glycerol) supplemented with protease inhibitors (0.5 mM PMSF, 0.025 mM N-CBZ-L-phenylalanine chloromethyl ketone, 0.025 mM N-p-tosyl-lysine chloromethyl ketone, and 0.025 mM L-1-tosylamide-2-phenyl-ethylchloromethyl ketone). After centrifugation at 4°C for 10 minutes at 10,000 ×g, the pellets were discarded and the supernatant was obtained.
After boiling for 5 minutes, we loaded 50 µg of protein onto a 12% SDS-polyacrylamide gel and performed electrophoresis at 120 V for 1.5 hours. The resolved proteins were transferred onto nitrocellulose membranes at 100 V for 2 hours. After incubation in a blocking buffer (5% non-fat milk, 0.05% Tween-20 in 20 mM TBS at a pH of 8.0) for 1 hour at room temperature, the blots were incubated overnight at 4°C with appropriate primary antibodies: AMH (1:400, sc-166752; Santa Cruz Biotechnology, Dallas, TX, USA), BCL-xL (1:100, sc-271121; Santa Cruz Biotechnology), BAX (1:300, sc-7480; Santa Cruz Biotechnology), A-SMase (1:200, ab83354; Abcam, Cambridge, UK), cleaved caspase-3 (1:500, 5a1e; Cell Signaling Technology, Danvers, MA, USA), and α-SMA (1:200, sc-53142; Santa Cruz Biotechnology).
In Figure 2, representative bands from the Western blot analysis are presented. Specifically, the bands for BCL-xL appear to be double, and this phenomenon is common for phosphorylated BCL-xL [22].
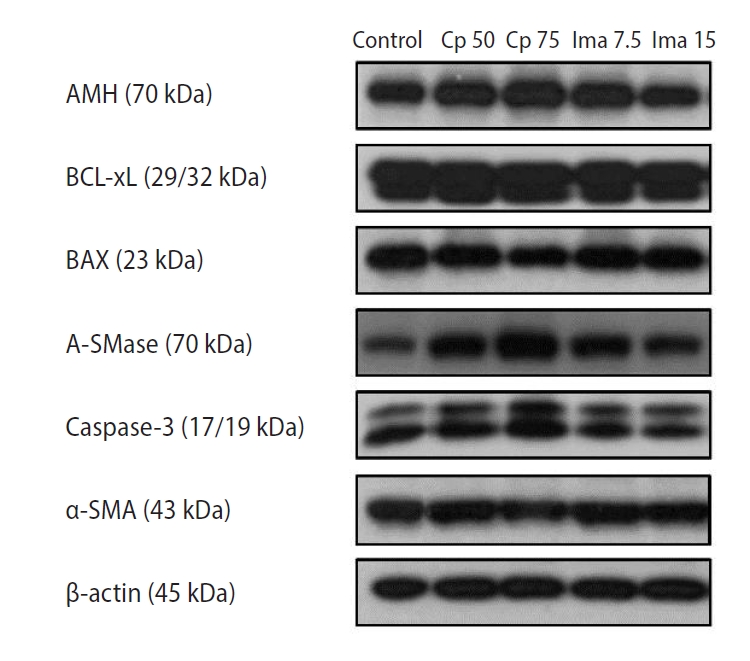
The expression levels of six proteins within the whole ovaries as determined by Western blots. Control, 0.1 mL of normal saline; Cp 50, cyclophosphamide 50 mg/kg; Cp 75, cyclophosphamide 75 mg/kg; Ima 7.5, imatinib 7.5 mg/kg; Ima 15, imatinib 15 mg/kg; AMH, anti-Müllerian hormone; A-SMase, acid sphingomyelinase; α-SMA, α-smooth muscle actin.
AMH is a well-known marker of ovarian reserve. BCL-xL is an anti-apoptotic marker and BAX is a pro-apoptotic marker. A-SMase is an enzyme known to increase the levels of the pro-apoptotic sphingolipid ceramide; thus, it acts as a pro-apoptotic marker. Caspase-3 is a well-known marker of late apoptosis. α-SMA, which is detected in tissues with disrupted blood vessels, is a marker of vessel damage [23].
Next, the blot was incubated with anti-rabbit secondary antibodies conjugated with horseradish peroxidase (1:1000, catalog #A4914; Sigma-Aldrich). Scion Image for Windows (Scion Corp., Frederick, MD, USA) was used to analyze the chemiluminescence signal. For each protein, eight or nine replicates were used. In the experimental groups, each protein level was expressed relative to the protein level of the saline control group. Therefore, the protein level of the saline control group was always 1.0.
5. Statistical analysis
We used IBM SPSS ver. 22.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism 5.0 (GraphPad, San Diego, CA, USA). Ovarian follicle counts were compared using one-way analysis of variance (ANOVA) followed by the Tukey multiple-comparison test. All samples were tested for the normality of the data distribution before ANOVA. A p-value <0.05 was considered to indicate statistical significance. The protein levels were compared using the Kruskal-Wallis test followed by the Mann-Whitney U test. In this analysis, the threshold for statistical significance was a p-value <0.01.
Results
The detailed ovarian follicle counts, including the proportion of G1 follicles in the five groups, are presented in Table 1. Notably, the number of primordial follicles was significantly lower in the group treated with imatinib 7.5 mg/kg (13.9±7.0 vs. 23.3±8.0, p=0.001), as well as in the group treated with imatinib 15 mg/kg (15.5±7.9 vs. 23.3±8.0, p=0.010) than in the control group. The Cp 75 mg/kg group also showed a lower number of primordial follicles than the control group (13.8±10.7 vs. 23.3±8.0, p=0.002), but the Cp 50 mg/kg group did not.

Ovarian subtype follicle counts and the proportions of G1 follicles in mice treated with Cp or imatinib
The ovarian follicle counts in the five groups are also depicted in Figure 3. The primary and antral follicle counts were all similar among the five groups (Figure 3B and D). The secondary follicle count was significantly lower in the imatinib 15 mg/kg–treated group than in the other four groups (p=0.001 for each) (Figure 3C).
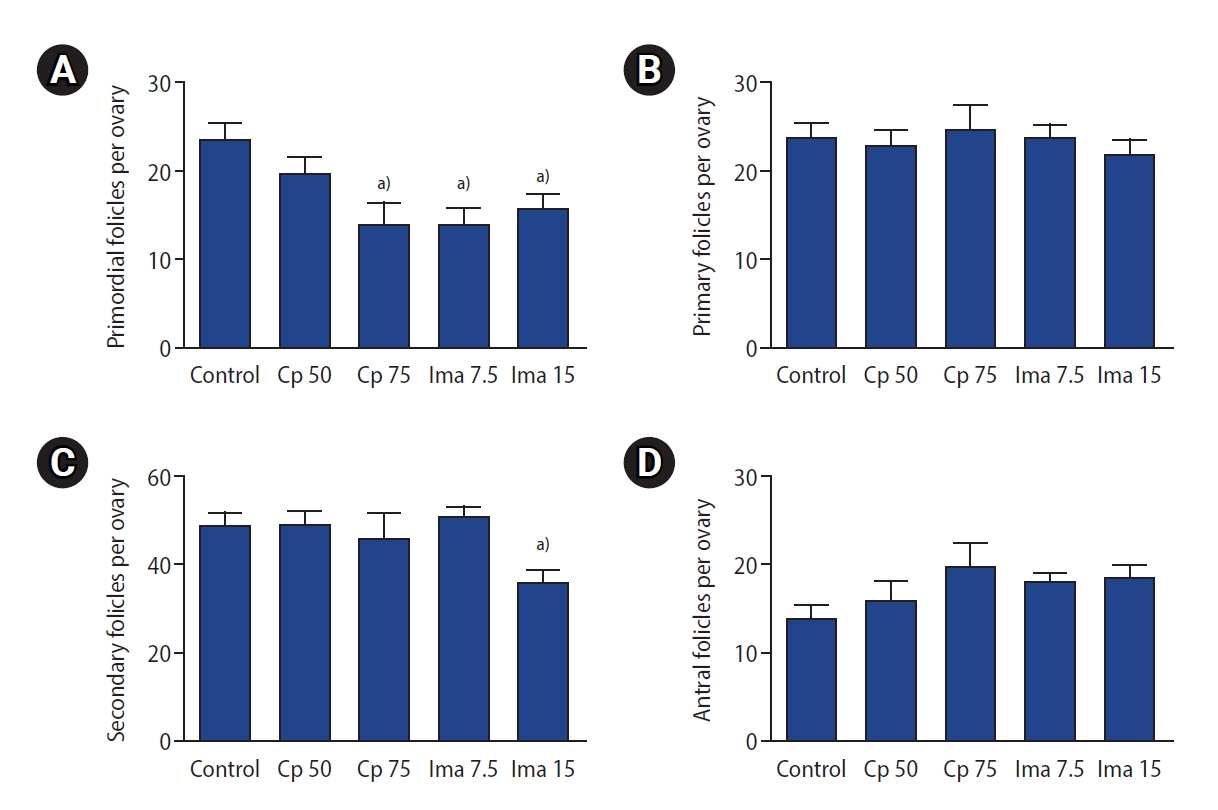
Ovarian subtype follicle counts in the five groups. Primordial (A), primary (B), secondary (C), antral (D) follicle counts. Values are presented as mean±standard error. Control, 0.1 mL of normal saline; Cp 50, cyclophosphamide 50 mg/kg; Cp 75, cyclophosphamide 75 mg/kg; Ima 7.5, imatinib 7.5 mg/kg; Ima 15, imatinib 15 mg/kg. a)p<0.05 by one-way analysis of variance followed by the Tukey multiple-comparison test.
The proportions of G1 follicles in the five groups are depicted in Figure 4. Although administration of imatinib (at a dose of either 7.5 or 15 mg/kg) significantly decreased the number of primordial follicles, the proportion of G1 primordial follicles was well preserved, as shown in Figure 4A. Similarly, the proportion of G1 primary and antral follicles was also preserved in the two imatinib-treated groups, but the proportion of G1 secondary follicles was not (Figure 4C). Interestingly, the administration of Cp at either dose (50 or 75 mg/kg) significantly decreased the proportion of G1 follicles in almost all types compared to the control group (Figure 4).
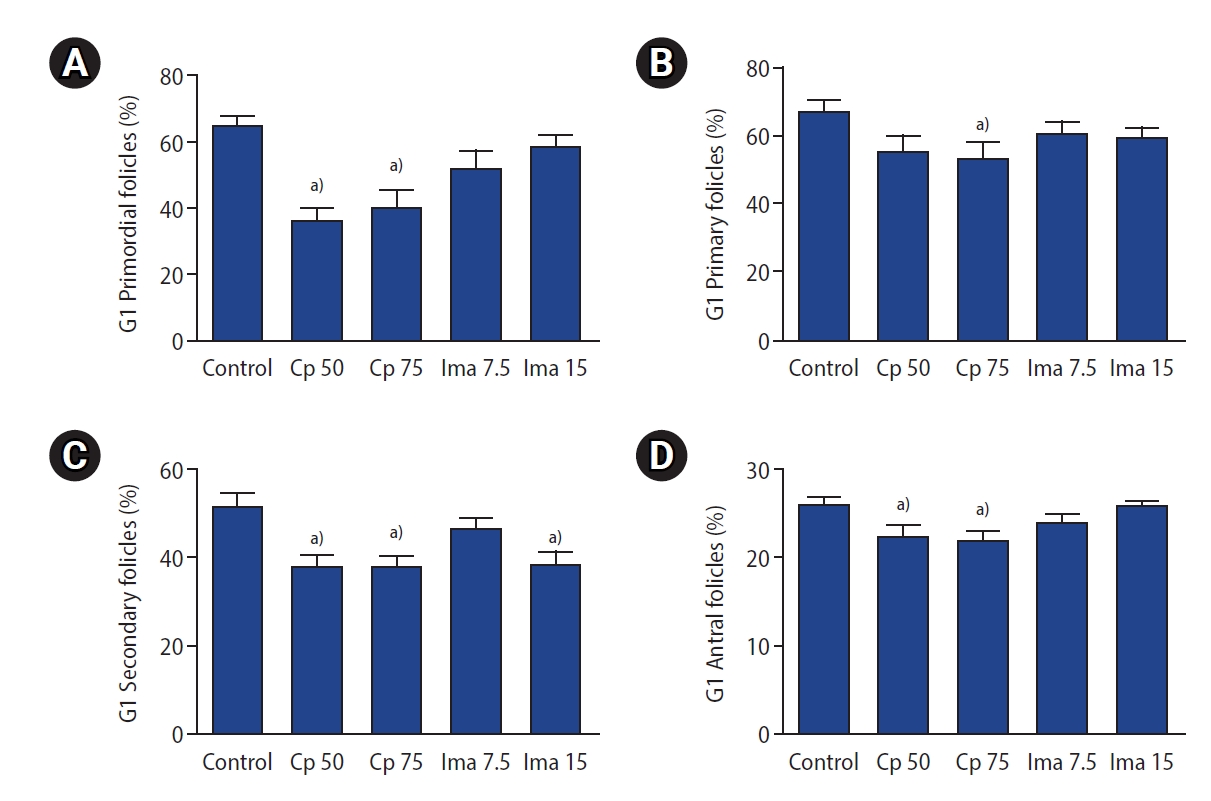
The proportion of good-quality (G1) subtype follicles in the five groups. G1 primordial (A), G1 primary (B), G1 secondary (C), G1 antral (D) follicles. Values are presented as mean±standard error. Control, 0.1 mL of normal saline; Cp 50, cyclophosphamide 50 mg/kg; Cp 75, cyclophosphamide 75 mg/kg; Ima 7.5, imatinib 7.5 mg/kg; Ima 15, imatinib 15 mg/kg. a)p<0.05 by one-way analysis of variance followed by the Tukey multiple-comparison test.
To summarize the findings regarding primordial follicles, administration of imatinib (7.5 or 15 mg/kg) significantly reduced the number of primordial follicles, but with good preservation of the proportion of G1 primordial follicles. The administration of Cp 50 mg/kg did not reduce the number of primordial follicles, but reduced the proportion of G1 primordial follicles. The administration of Cp 75 mg/kg significantly reduced both the number of primordial follicles and the proportion of G1 primordial follicles.
The levels of the six ovarian proteins in the five groups are depicted in Figure 5. The ovarian AMH levels in the groups treated with Cp 50 mg/kg, Cp 75 mg/kg, and imatinib 15 mg/kg were similar to those in the control group, but the AMH level was rather high in the imatinib 7.5 mg/kg group (p<0.001). The ovarian levels of anti-apoptotic BCL-xL and pro-apoptotic BAX and A-SMase were similar in all five groups. However, ovarian caspase-3 protein levels were significantly higher in the groups treated with Cp 50 mg/kg, Cp 75 mg/kg, imatinib 7.5 mg/kg, and imatinib 15 mg/kg than in the control group. In addition, α-SMA levels were significantly higher in the groups treated with imatinib or Cp than in the saline control group.
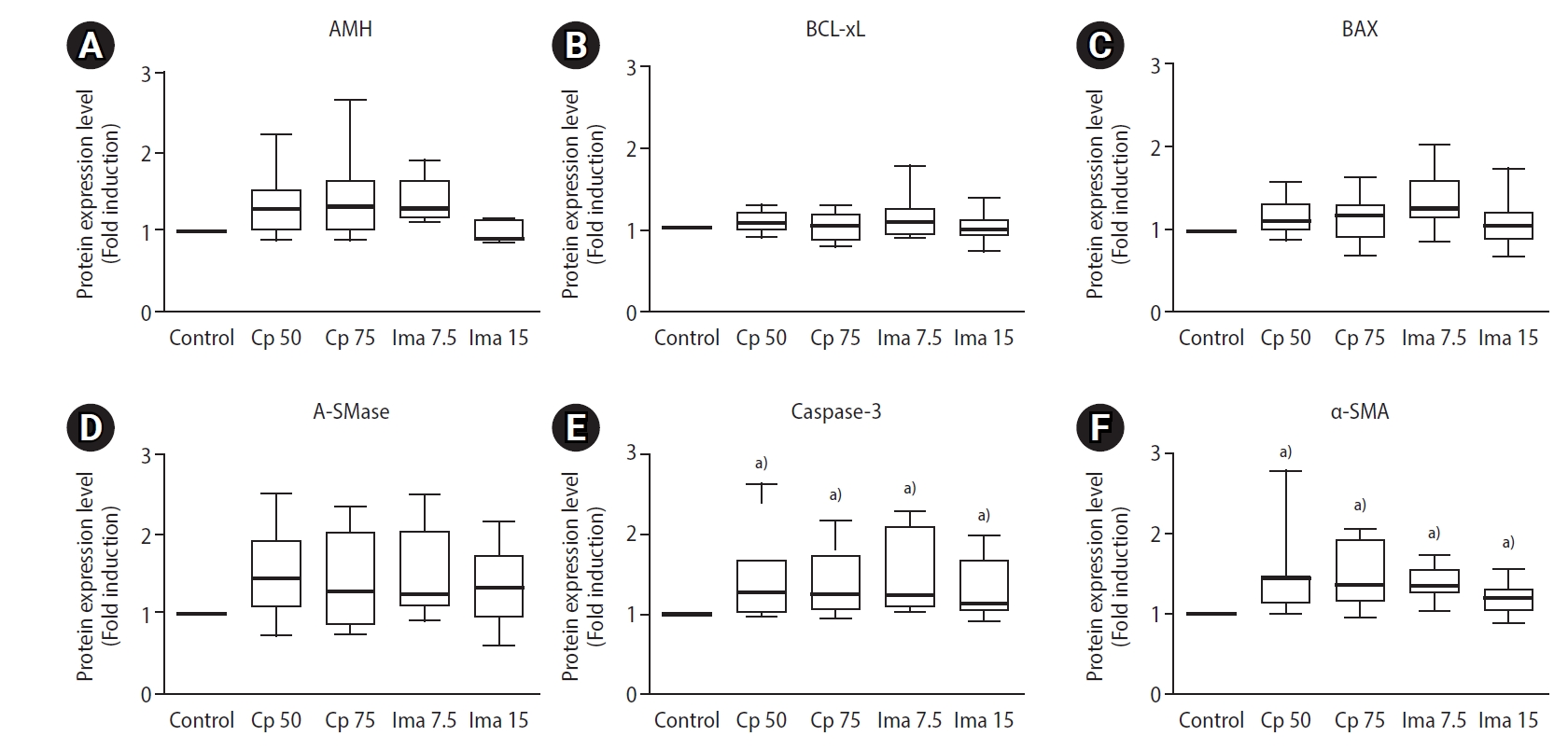
Box-whisker plots to demonstrate relative protein levels within whole ovaries. The ovarian levels of anti-Müllerian hormone (AMH, A), BCL-xL (B), BAX (C), acid sphingomyelinase (A-SMase, D), caspase-3 (E), and α-smooth muscle actin (α-SMA, F). Control, 0.1 mL of normal saline; CP 50, cyclophosphamide 50 mg/kg; CP 75, cyclophosphamide 75 mg/kg; Ima 7.5, imatinib 7.5 mg/kg; Ima 15, imatinib 15 mg/kg. a)p<0.01 when compared to the saline control group by the Mann-Whitney U-test.
Discussion
In the present study, we examined the ovotoxicity of imatinib as well as Cp in a mouse model. Administration of imatinib 7.5 and 15 mg/kg significantly reduced the number of primordial follicles, an indicator of ovarian reserve. Increased ovarian caspase-3 and α-SMA levels were observed in imatinib-treated mice. These findings suggest that imatinib induces primordial follicle loss, possibly via an apoptotic mechanism involving caspase-3 and vascular damage.
A decreased number of primordial follicles was also observed in the Cp 75 mg/kg group, while increased ovarian caspase-3 levels were observed in both the Cp 50 and 75 mg/kg groups. Caspase-3 is a major downstream effector enzyme in the late apoptosis process, and it has also been reported that Cp induces increased levels of caspase-3 [24].
Studies have reported that imatinib induces the loss of primordial follicles in animal models, which is consistent with our findings [14-16]. Furthermore, we here found, for the first time, that imatinib exerts an ovotoxic effect through an apoptotic mechanism involving caspase-3, similarly to Cp. Given that there was no difference in ovarian expression of BCL-xL, BAX, and A-SMase between the imatinib- or Cp-treated groups and the control, it can be inferred that BCL-xL, BAX, and A-SMase do not seem to take part in the apoptotic mechanism that causes primordial follicle reduction.
Imatinib has been proposed as an agent to prevent primordial follicle loss caused by cisplatin [19]. This ovoprotective effect was presumed to derive from the fact that imatinib could inhibit the c-Abl-TAp63 pathway, which is one of the main mechanisms of cisplatin-induced follicular apoptosis. However, subsequent studies have challenged these results, and the potential ovoprotective effect of imatinib remains a topic of debate. Therefore, since our study demonstrates imatinib’s ovotoxicity, caution is needed regarding the use of imatinib to protect against ovarian damage induced by anticancer drugs.
The so-called “burnout” hypothesis has been suggested as a mechanism for ovarian damage induced by Cp [25,26]. According to this hypothesis, Cp inhibits the dormancy of primordial follicle pool by destroying growing follicles. This induces premature activation of primordial follicles, thereby reducing the number of primordial follicles. We found in this study that Cp reduced the primordial follicle count without affecting the primary, secondary, or antral follicle count. Therefore, Cp does not seem to cause “burnout” phenomenon, at least at the doses we studied. Luan et al. [24] also demonstrated that Cp specifically depletes primordial follicles by directly inducing apoptotic cell death, rather than depleting the primordial follicle pool by activating or destroying growing follicles.
In the present study, we also found that imatinib reduced the primordial follicle count, but did not affect other growing follicle counts. Analogously, imatinib does not seem to induce the “burnout” phenomenon, at least at the doses we studied. We found that imatinib reduced the primordial follicle count, similarly to Cp. However, the extent of primordial follicle damage by imatinib might be modest in comparison with the damage caused by Cp, because the proportion of G1 follicles was maintained in the imatinib-treated groups. This observation could be clinically important, because the ovarian reserve may recover after discontinuing imatinib if G1 follicles are maintained during imatinib administration. Further studies are needed to determine how long the decrement of primordial follicles lasts after imatinib administration and when the number of primordial follicles recovers after imatinib discontinuation.
In the present work, ovarian AMH levels were well preserved after 1 week of exposure to imatinib or Cp. This could be explained by the good preservation of primary and secondary follicles after imatinib or Cp administration, since they are the main sources of AMH production. Meanwhile, it has been reported that serum AMH levels dropped within 3 days after Cp administration, and then rebounded to equivalent levels 7 days after Cp treatment [24]. Those researchers found that healthy granulosa cells in follicles replaced damaged granulosa cells and secreted AMH. Further research is needed to clarify how ovarian AMH levels change over time after imatinib administration.
Our study has several limitations. Although we demonstrated the ovotoxicity of imatinib, the underlying mechanism of imatinib-induced ovotoxicity should be further elucidated. We exposed mice for 1 week via a single dose of imatinib. Further studies are needed to evaluate the effects of various exposure durations and doses of imatinib. In conclusion, imatinib administration to mice negatively affects the primordial follicle count. This detrimental effect might be induced via caspase-3-dependent apoptosis and vascular damage, similarly to Cp. The potential ovotoxicity induced by imatinib treatment should be further studied both biologically and clinically.
Notes
Conflict of interest
Byung Chul Jee has been the editor-in-chief of Clinical and Experimental Reproductive Medicine since 2018; however, he was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflict of interest relevant to this article was reported.
Author contributions
Conceptualization: SJK, BCJ. Data curation: SJK, BCJ. Formal analysis: all authors. Methodology: SJK, BCJ. Project administration: SJK, BCJ. Visualization: SJK. Writing-original draft: all authors. Writing-review & editing: all authors.