High efficiency of homemade culture medium supplemented with GDF9-β in human oocytes for rescue in vitro maturation
Article information
Abstract
Objective
Optimizing culture media for the incubation of immature oocytes is a vital strategy to increase the oocyte maturation rate during in vitro maturation (IVM) programs. This study evaluated the IVM and fertilization rates of human germinal vesicle (GV) and metaphase I (MI) oocytes using two different maturation media (commercial and homemade) with or without growth differentiation factor 9-β (GDF9-β). supplementation.
Methods
Immature oocytes from intracytoplasmic sperm injection (ICSI) cycles were collected and assigned to one of two IVM culture media (commercial or homemade; cleavage-stage base). After maturation, MII oocytes were examined under an inverted microscope for the presence of the polar body, zona pellucida (ZP) birefringence, and meiotic spindle (MS) visualization after maturation in four conditions (commercial or homemade medium, with or without GDF9-β. ICSI was done for matured oocytes, and fertilization was confirmed by the visualization of two distinct pronuclei and two polar bodies.
Results
No significant differences were found between the two culture media in terms of the time and rate of oocyte maturation or the rate of fertilization (p>0.05). Growth factor supplementation increased the 24-hour maturation rate for both GV and MI oocytes only in homemade medium. The maturation rate after 24 hours was higher for MI oocytes (p<0.05). Similar results were observed for MS visualization and ZP structure in both types of media (p>0.05).
Conclusion
Higher rates of oocyte maturation and fertilization were observed after application of homemade medium supplemented with GDF9-β. Therefore, this combination may be recommended as an alternative for clinical IVM programs.
Introduction
In recent years, in vitro fertilization (IVF) cycles have achieved considerable success rates. However, IVF is accompanied by the risk of major complications of multiple pregnancies and ovarian hyperstimulation syndrome, a potentially life-threatening condition. The only way to prevent this syndrome is to eliminate ovarian triggering by hormonal administration [1]. One strategy is to perform in vitro maturation (IVM) of immature oocytes as an alternative to exogenous hormonal protocols [2]. Since IVM does not require ovarian stimulation, it has received more attention in recent years [1]. Patients with polycystic ovary syndrome (PCOS), unexplained infertility, poor quality embryos, normal ovulation, a poor response, and a need for fertility preservation are suitable for IVM. The rescue of oocytes that do not successfully mature during stimulated cycles is another important benefit of this technique [3].
IVM is not a new procedure, and the first IVF procedure was done using an immature rabbit oocyte [4]. Some types of IVM are rescue IVM [5], conventional or standard IVM, and biphasic IVM [4]. Rescue maturation is motivated by the fact that approximately 15% of oocytes from conventional IVF cycles are immature [6,7]. Rescue IVM has shown very limited success; hence, this technique is not routinely used [8]. However, improving the quality of IVM oocytes would be of great clinical value for maximizing the number of mature oocytes and embryos, especially in poor responders [9,10].
Two major types of IVM media exist: commercial and homemade. Homemade medium is prepared from various base media, which are routinely used in IVF laboratories, such as human tubal fluid (HTF) [1], culture medium 199 [11,12], or cleavage-stage [13] and embryo-stage medium [14]. Since cleavage and embryo media are used for the aim of IVM, in addition to their defined use as embryo culture, they are also considered homemade. Previous studies have compared the efficiency of different culture media to optimize human oocytes in IVM conditions [1,10,15]. In a study by Fesahat et al. [10], the efficacy of four different culture media to promote human metaphase I (MI) oocyte maturation was compared. The rates of fertilization and embryo development were also evaluated in oocytes undergoing IVM in stimulated cycles. They concluded that various types of commercial media did not lead to more success in maturation, fertilization, and embryo development for MI oocytes than homemade media. In another study, tissue culture medium 199 and HTF media were compared concerning the human maturation, fertilization, and embryo quality rates, and it was found that, despite the widespread use of HTF in IVF laboratories, it was unsuitable for the immature oocytes retrieved from PCOS patients [1]. In this regard, the effects of supplementation with different growth factors have been studied. In a recent study, Chatroudi et al. [16] investigated the effect of human IVM medium supplementation with growth differentiation factor 9 (GDF9) and cumulus cells (CCs). Their results showed that supplemented medium enhanced fertilization and embryo formation rates, as well as the viability of blastocysts from vitrified cleavage embryos. Other studies also reported promising effects of GDF9 supplementation in enhancing the IVM results in mice [2] and pigs [17]. GDF9, which is secreted by oocytes, is capable of stimulating CC expansion in vivo and, during CC expansion, it promotes the hyaluronic acid-rich matrix [18]. It was reported that the morphology of both the oocyte and the surrounding CCs, especially the size of CCs and the amount of expansion, is the most important criterion for IVM [16]. In the study by Fesahat et al. [19], homemade medium was supplemented with human follicular fluid, but it might be easier to make and handle homemade media with GDF9.
Successful meiotic maturation of oocytes plays a crucial role in normal fertilization and subsequent embryo development [20,21]. Therefore, the maturation process and the choice of medium are critical steps in IVM technology [1]. Applying formulated commercial media has been reported to have several disadvantages, such as a short shelf life and high cost when compared with the standard medium that is used at fertility centers [10]. The aim of the current study was to compare the outcomes of IVM in terms of maturation, duration of maturation, oocyte quality, and fertilization rates between two IVM culture media: cleavage-based medium and commercial medium. Since there is insufficient evidence regarding the effects of GDF9-β IVM medium supplementation on the development and quality of IVM oocytes, we supplemented both types of culture media with this factor.
Methods
This study was approved by the Research Ethics Committee of Rafsanjan University of Medical Sciences, Rafsanjan, Iran (IR.RUMS.1399.241), and followed the Helsinki Declaration of 1975. All procedures involving human participants were done in accordance with the standards and ethical rules of the Yazd Fertility Center. All patients provided written informed consent for participation.
Sibling immature oocytes were collected from 82 intracytoplasmic sperm injection (ICSI) cycles between December 2017 and July 2019. The inclusion criteria were male factor infertility and women aged <38 years, with at least four immature oocytes. Four immature sibling oocytes were assigned to four main groups: commercial and homemade media with and without GDF9-β. The exclusion criteria were endometriosis, PCOS, and severe male factor infertility.
1. Ovarian stimulation protocol
All patients underwent the multiple-dose gonadotropin-releasing hormone (GnRH)-antagonist controlled ovarian hyperstimulation protocol with recombinant follicle-stimulating hormone (rFSH; Cinnal-f, Cinnagen, Tehran, Iran) or Gonal-f (Merck Serono, Geneva, Switzerland) started on the second day of the menstrual cycle. Once the dominant follicle (13–14 mm) was detected by sonography, a GnRH antagonist (Cetrotide; Serono international, Geneva, Switzerland) was initiated, and rFSH was continued up to the day of ovulation triggering. Recombinant human chorionic gonadotropin (hCG; PD preg: Pooyesh Darou, Tehran, Iran) was administered for final maturation, when at least one follicle reached a diameter of 18 mm, 36 hours prior to oocyte retrieval.
2. IVM medium
A commercial medium (MediCult IVM System, Origio, Denmark) and a homemade medium were used for IVM. The base for the homemade medium was G2 (Vitrolife, Gothenburg, Sweden), which is used for the culture of human embryos from the 8-cell stage to the blastocyst stage [10]. The homemade medium was supplemented with 75 mIU/mL FSH and 75 mIU/mL luteinizing hormone (LH; Ferring Pharmaceuticals, Suffern, NY, USA). The commercial medium had two parts: LAG medium (vial 1) and IVM medium (vial 2). Vial 2 was supplemented with an hCG solution (100 mIU/mL), an FSH solution (Ferring Pharmaceuticals, Suffern, NY, USA; 75 mIU/mL), and human serum albumin. According to the use of growth factor supplementation (GDF9‑β, recombinant human factor 9, Sigma-Aldrich, St. Louis, MO, USA), there were four groups of IVM culture media: cleavage medium (G2) and commercial medium, with and without GDF9‑β.
3. Oocyte collection and IVM culture
Approximately 2–3 hours after ovarian puncture, the cumulus-oocyte complexes were denuded using both enzymatic exposure (80 IU/mL hyaluronidase; Irvine Scientific, Santa Ana, CA, USA) and mechanical pipetting. The denuded oocytes were then checked for nuclear maturity. However, between the germinal vesicle (GV) and MI stages, GV breakdown (GVBD) and the circular bivalent stage (absence of a polar body [PB]) occur. Since these two stages cannot be recognized using polarizing optics or birefringence from the GVBD phase, an oocyte without GV or a PB is considered as MI-arrested. In the literature, GVBD is mentioned as the first visible event of oocyte meiotic maturation, followed by chromosome condensation and alignment at the MI plate. These oocytes are MI oocytes [22,23]. Both GV and MI oocytes were collected and assigned into four main culture groups; homemade medium without GDF9‑β, homemade medium with GDF9‑β (200 ng/mL) [2,16], commercial medium without GDF9‑β, and commercial medium with GDF9‑β. Thus, there were a total of eight subgroups, as shown in Figure 1.

Flowchart of the immature oocytes assigned groups. MI, metaphase I; GV, germinal vesicle; GDF9-β, growth differentiation factor 9-β.
For IVM with G2 medium, four immature oocytes were cultured in 25-µL droplets of the medium in a triple gas incubator with 5% CO2, 5% O2, and 90% air, under mineral oil (MediCult,, Lyon, France). For the commercial medium, according to the manufacturer’s instructions, four-well plates were used: the two superior wells for vial I (LAG medium) and the two inferior wells for vial II (IVM medium). After oocyte pick-up, the immature oocytes were incubated in 0.5 mL of LAG medium in the superior wells, in a CO2 environment at 37°C for 2–3 hours prior to transfer to the final maturation medium. Each group of oocytes was then transferred into 0.5 mL of IVM medium in the two inferior wells of the four-well culture dish and incubated for 24 or 48 hours.
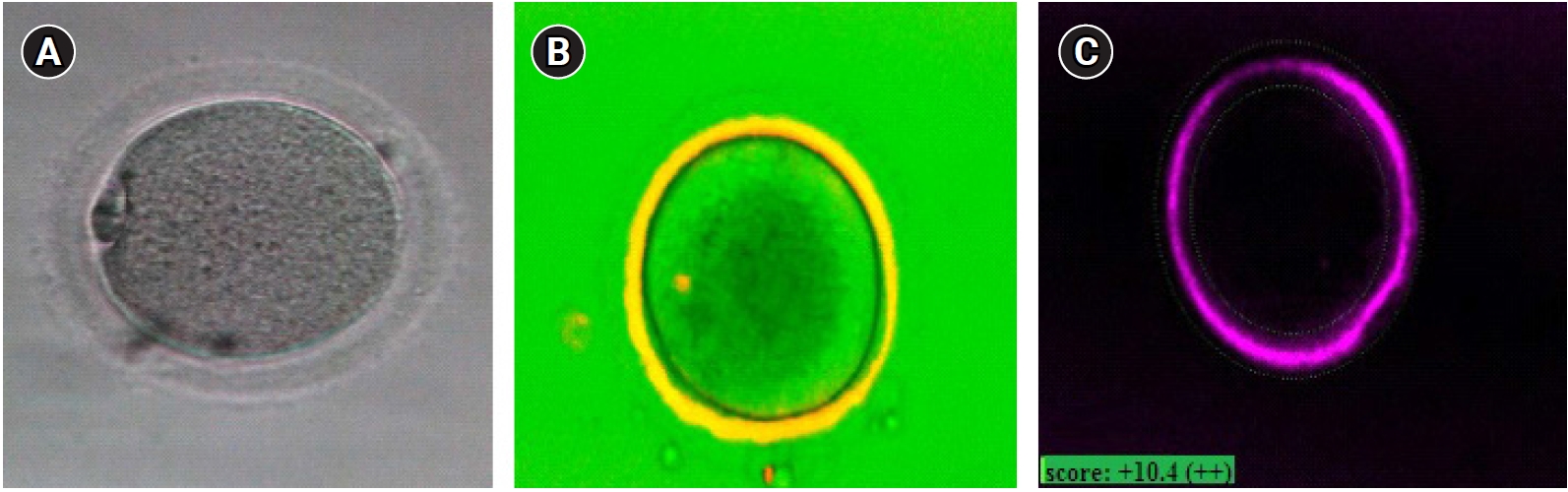
Oocyte maturity was confirmed under an inverted microscope (Nikon Co, Tokyo, Japan), with the presence of the first PB in the perivitelline space (Figure 2A). If the oocyte was mature, PolScope imaging was applied to analyze the zona pellucida (ZP) birefringence and meiotic spindle (MS). However, immature oocytes were left in the incubator for another 24 hours. At the end of 48 hours, the oocytes were checked for maturity. Mature oocytes were injected according to the standard ICSI protocol, and then 3–4 injected oocytes were cultured in a single drop of culture medium (20 µL droplets of G-1 Medium; Vitrolife), under mineral oil.
4. PolScope imaging and ICSI
An inverted microscope (TE300, Nikon) equipped with a polarizing optical system (OCTAX polarAIDE, Octax) was used for PolScope imaging. A droplet of buffered medium (G-Mops-V1; Vitrolife, 5 mL) was placed in a glass-bottomed dish (WillCo-dish; Bellco Glass, Vineland, NJ, USA) covered with mineral oil (Irvine Scientific). After maturation, the MII oocytes were loaded in the mentioned droplets and scored for ZP birefringence and MS visualization. For ZP birefringence, green was considered as high quality, yellow as moderate quality, and red as low quality (Figure 2B and C). Following oocyte morphology assessment, the ICSI procedure took place [24]. Sixteen hours after ICSI, normal fertilization was confirmed by the visualization of two distinct pronuclei and two PBs under an inverted microscope (Nikon). Since the institutional policy was not to transfer the embryos generated from IVM, all zygotes were discarded due to ethical issues.
5. Statistical analysis
Since most of the findings were categorical data, the chi-square test was performed for statistical analyses. When the data frequency was less than 5%, the Fisher exact test was used. The fertilization rates between groups were compared using the chi-square test. Multinomial logistic regression was used for dependent variables with more than two categories. IBM SPSS ver. 20 (IBM Corp., Armonk, NY, USA) was applied for data analysis, and a p-value <0.05 was considered as indicating statistical significance. In each table, p-values from the chi-square test are reported for analyses of the relationships between two variables, whereas for analyses with three or more variables, p-values were calculated using multinomial logistic regression, whenever the result from the chi-square test was significant.
Results
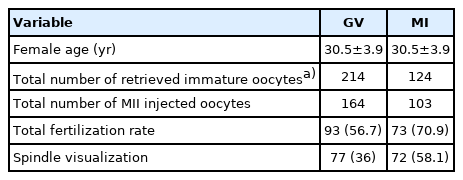
The descriptive statistical analysis is presented in Table 1. Data from 124 ICSI cycles were gathered, of which 82 met our criteria and had at least 4 immature oocytes. In these cases, a total of 885 oocytes were retrieved, including 338 immature (214 GV and 124 MI) and 547 mature oocytes. A total of 82 ICSI cycles underwent IVM. The chi-square test did not show a significant difference in the distribution of oocytes between homemade and commercial media (Table 1). After IVM, 267 oocytes were matured and 71 oocytes remained immature or degenerated. After 48 hours of IVM, the total maturity rate was 81.5% for the homemade medium and 76.4% for the commercial IVM medium. However, there was no significant difference between the total conversion rate (after 48 hours) between the commercial and homemade medium (p=0.27) (Table 2). Multinomial logistic regression showed a higher 24-hour maturation rate for MI than for GV oocytes (p=0.04) (Table 2). Multinomial logistic regression also demonstrated a higher maturation rate after 24 hours for both GV and MI oocytes when homemade IVM medium was supplemented with GDF9‑β. However, no significant difference was observed for commercial medium (Tables 3 and 4).

Comparison of the maturation rate between commercial and homemade medium and between GV and MI oocytes

Comparison of the maturation rate between homemade and commercial media, with and without growth factor supplementation, for GV oocytes

Comparison of the maturation rate between homemade and commercial IVM media, with and without growth factor, for MI oocytes
The chi-square test showed no significant differences in ZP birefringence between the two types of IVM media (p=0.24) or between MI and GV oocytes (p=0.11). However, high-quality results for ZP birefringence were found more frequently when GDF9‑β was added to the culture medium (p=0.01) (Figure 3). The spindle was visible in 55.8% of the matured oocytes. The chi-square test showed that the spindle was more frequently visible (p=0.01) when MII oocytes were derived from MI oocytes (65%) than from GV oocytes (50.0%). However, there were no significant differences between the two types of culture media regarding MS visualization when GDF9‑β was added to the culture medium (p=0.17). The fertilization rate was higher in MI oocytes than in GV oocytes, as well as in 24-hour matured oocytes in comparison to 48-hour matured oocytes (p<0.05). The fertilization rate showed no significant difference according to the type of culture medium or GDF9‑β supplementation. However, the rate was higher when the spindle was visible in the matured oocyte (p<0.05) (Table 5). There was no correlation between the fertilization rate and ZP quality.
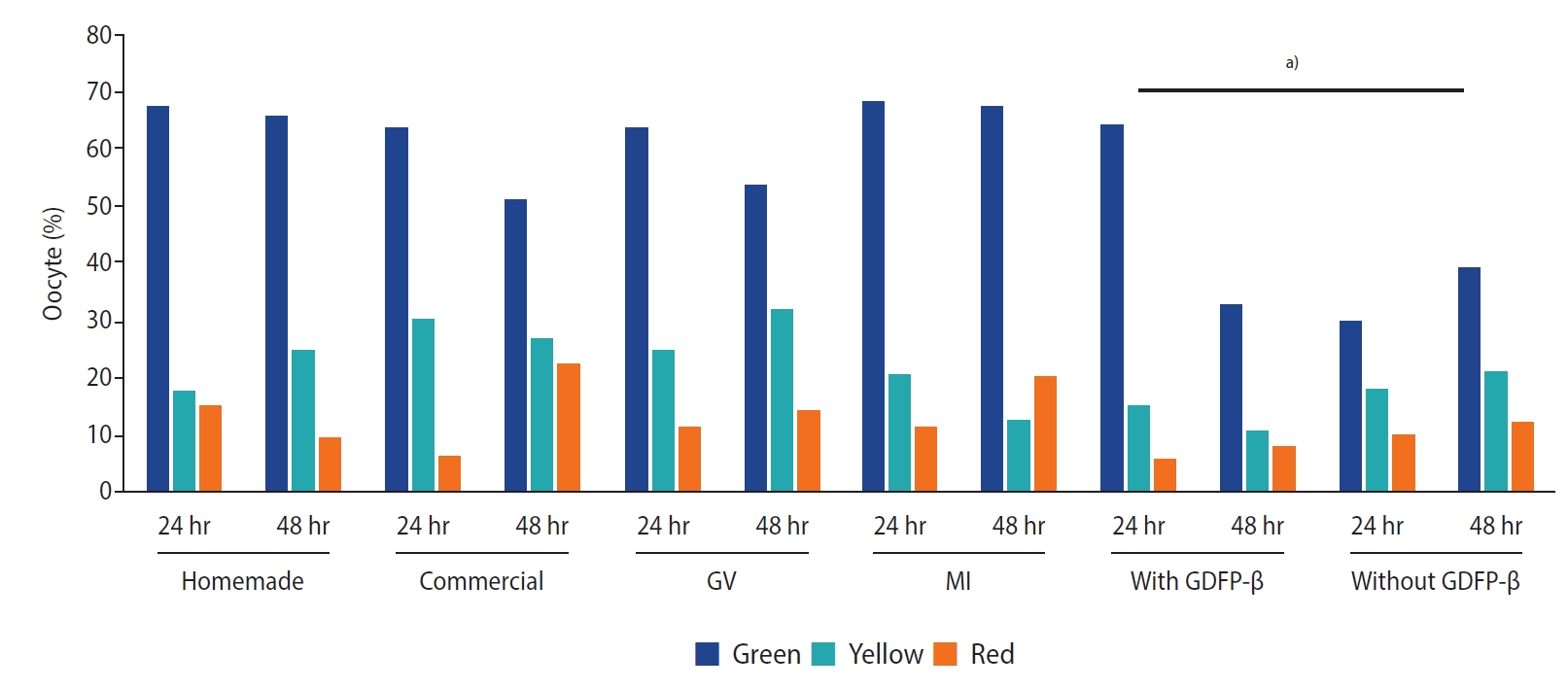
Comparison of zona pellucida birefringence between two types of in vitro maturation medium, and germinal vesicle (GV) and metaphase I (MI) oocytes, with and without growth differentiation factor 9-β (GDF9-β). a)There were no significant differences in zona pellucida birefringence between the two kinds of IVM medium nor between MI and GV oocytes, but the green score was higher when GDF9‑β was added to the culture medium.
Discussion
After 24 hours of incubation, the total maturity rate was 79.9% for the homemade IVM medium and 76.4% for the commercial IVM medium. The IVM success rate has been reported from 16.4% to 88.3%, depending on the time of incubation [25]. Possible reasons for this wide range may include the use of different types of IVM, with full or mild stimulation and with or without hCG priming, as well as differences in the cause of infertility, the medium used, and medium supplementation. No significant differences were found between the two types of media regarding the maturation rates. This finding is in accordance with previous studies that reported no significant advantage of commercial IVM media over standard media [10,26]. Furthermore, some good outcomes have been reported for non-commercial media; for instance, in this study, we used G2 medium, a routinely used medium, as the base medium for IVM. The limited expiration date of commercial IVM media is one of their disadvantages; moreover, they are costly and not routinely used in contrast to standard IVF media.
Our results showed no significant differences between the GV and MI maturation rates after 48 hours, but the maturation rate was higher in MI oocytes after 24 hours of culture. This may be due to the fact that MI oocytes are more developmentally advanced than GV oocytes. Other studies also showed that an extended duration of IVM is accompanied by better clinical outcomes [25,27]. It is well established that immature oocytes from different developmental stages need different timing to reach maturation [25]. However, oocytes that do not reach maturation may need more time to mature or may be subject to certain inherent errors in meiotic maturation [28,29]. Therefore, caution is needed when using later-matured oocytes in the assisted reproductive technology cycles, as studies have shown that converting faster to MII is accompanied with better embryonic development in an IVM program [30].
The addition of oocyte-specific factors during IVM is a relatively new concept. Our results showed a higher maturation rate after 24 hours for both GV and MI oocytes when homemade IVM medium was supplemented with GDF9‑β, whereas GDF9‑β supplementation did not have a significant impact in commercial medium. This finding is in line with the study of Yeo et al. [2], according to which oocytes’ developmental competence decreased in IVM. This may be due to oocyte–CC communication disruption and inappropriate levels of oocyte factors such as GDF9‑β. They also reported that IVM supplementation with exogenous GDF9‑β was accompanied by better embryo development and fetal viability [2]. In this regard, Chatroudi et al. [16] showed that IVM medium supplementation with GDF9‑β increased fertilization and embryo formation, as well as enhancing blastocysts’ viability after embryo cryopreservation. In 2006, Hussein et al. [31] showed that during bovine IVM, GDF9‑β or bone morphogenetic proteins 15 enhanced oocyte developmental potential and consequently blastocyst formation. The results of this study confirm these effects in human IVM programs. A point that must be addressed is that the positive effect of GDF9‑β was found only in homemade medium, but not in commercial medium. This may have been because of interactions between this growth factor and those found in the commercial medium. Unfortunately, the biological role of GDF9‑β and other oocyte-specific factors in IVM is still unclear. Clarifying this issue would be of great importance for understanding the fundamental process of oocyte maturation and development to improve IVM media and thereby increase IVM-ICSI success rates. Another reason for this positive effect of GDF9 in homemade medium, but not commercial medium, may be the fact that commercial media are more complex than homemade media, which are made in-house. Commercial media, which are prepared with high quality control and standardized formulations, create an optimal environment for the immature oocytes, with no need for supplementation [32].
Regarding the ZP structure, no significant differences were found according to the type of culture medium or maturation timing (24 or 48 hours). The only difference was related to GDF9‑β supplementation, which led to a higher frequency of high-quality (green color) findings. The MI and GV oocytes had the same ZP quality score. No previous study has compared ZP birefringence between GV and MI oocytes in IVM programs. Omidi et al. [9] reported that the ZP structure was not influenced by IVM process, but according to other studies that evaluated the effect of GDF9 supplementation, the division and expansion of CCs are thought to constitute a mechanism explaining growth factor efficacy [16]. It has also been reported that ZP is synthesized by both oocytes and granulosa cells [33], and GDF9 may affect the ZP score through the regulation of granulosa cells.
Although the results from PolScope showed no signs of ZP structure disruption during IVM, scanning electron microscopy of caprine oocytes showed a higher frequency of the mature type II ZP surface in the ovulated oocytes than in IVM oocytes. The authors concluded that in a caprine model, the ZP structure is related to oocyte maturity [34]. Our results also showed more ZP marked in green (indicating high quality) when the culture medium was supplemented with GDF9‑β. This is the first time that the ZP structure has been evaluated in conditions of IVM medium supplemented with GDF9‑β. However, previous studies reported better human IVM outcomes with exogenous GDF9‑β and concluded that it might be a promising approach for IVM [2,16].
The total MS visualization rate was 55.8%, while other studies reported a range from 51.9% to 73.8% [35,36]. The MS is composed of microtubules that can be temporarily depolymerized by a decrease in temperature, a prolonged process of visualization, or a pH change [37]. Our results showed no significant differences regarding spindle visualization between the two types of culture media, or when the maturation medium was supplemented with GDF9‑β, but there was a higher likelihood of spindle detection in the MI-matured oocytes. This finding is in line with the study by Braga et al. [36], which showed that the spindle was more visible when MII oocytes were derived from MI rather than GV oocytes. More spindle detection was reported in in vivo matured than in IVM oocytes [38]. It has also been suggested that the IVM process may have detrimental effects on MS organization [39]. A prior study reported that spindle properties may be influenced by variables such as oocyte manipulation [40]. The results showed that GV- matured in vitro oocytes had impaired maturation and lower spindle detection in comparison to MI-stage oocytes. The nuclear maturation process consists of GVBD, meiosis resumption, and extrusion of the first PB. It is thought that during the IVM process, the in vitro environment may have an important effect on the human oocyte`s maturation potential. MI oocytes are developmentally more advanced than GV oocytes, as they reach a higher level of maturity during the first 24 hours.
Higher fertilization rates were recorded from MI-matured than from GV-matured oocytes. Another study likewise reported fertilization rates of 69% and 53% after maturation of MI and GV oocytes, respectively. The reason for the higher fertilization rate for MI oocytes may be the fact that MI oocytes are more developed and need a shorter time for maturation. Immature oocytes that matured during the first 24 hours had a higher fertilization rate than those that required 48 hours of maturation. Another study also showed that a prolonged incubation time (up to 48 hours) did not improve the fertilization rate of IVM oocytes [10]. In the literature, 24–30 hours of incubation is described as the ideal time for IVM outcomes [41,42]. It was shown that GV oocytes that matured during 30 hours of incubation were more competent for later development than those needing a longer time. Faster maturation was accompanied by better embryonic development [30]. Thus, although a longer time may allow a more mature nucleus to be achieved, this does not necessarily indicate oocyte cytoplasmic competence. Furthermore, compromised reproductive outcomes in IVM transfer cycles [43] may be a consequence of low cytoplasmic competency in the matured oocytes during IVM. Much progress has recently been made regarding IVM efficacy, including biphasic IVM with pre-IVM culture, in which the aim is to achieve more cytoplasmic competency. In this new method, with a pre-IVM step, surrounding CCs are kept for paracrine signaling, and this method also tries to maintain the oocyte (GV) in a meiotically arrested stage. Meanwhile, by creating conditions similar to the post-LH surge follicular environment for the initiation and progression of meiosis, the achievement of developmental competence of the oocyte during 24 hours is facilitated. This method has potential for increasing the rates of synchronous maturation and MII formation [44,45].
Higher fertilization rates were seen when the spindle was visible before ICSI. This finding is in line with other studies that showed a higher fertilization rate when the MS was observed [36,39]. It is believed that the spindle and chromosome alignment of immature oocytes can be affected by the IVM process. S disturbance and the subsequent abnormal chromosomal organization result in embryo aneuploidy [37]. There was no significant difference in the fertilization rate between the two types of culture media, and there was also no significant correlation between the fertilization rate and ZP score. Ashourzadeh et al. [24] also showed no correlation between the ZP score and the fertilization rate in an IVM program.
In conclusion, a commercial medium designed especially for IVM oocytes with a standardized formulation was associated with good outcomes. Homemade culture medium supplemented with GDF9‑β can also be used as a substitute IVM medium. MI oocytes are advanced in terms of both nuclear and cytoplasmic development compared to GV oocytes. In addition, IVM oocytes with a PB extrusion may not be parallel with cytoplasmic maturation, so caution must be used when performing rescue IVM of GV oocytes in clinical practice. Among various approaches for morphologic characterization, MS visualization is considered a powerful criterion for the prediction of normal oocytes. A limitation of this study is the lack of data regarding embryo development. It must be mentioned that our policy was not to transfer IVM-derived embryos in ICSI cycles, so the zygotes were discarded after checking for two pronuclei for ethical reasons.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Author contributions
Conceptualization: MM, MV. Data curation: FA, MV. Formal analysis: MV. Methodology: MV, MAK. Project administration: MV. Visualization: MM, FA. Writing– original draft: MV, MAK. Writing–review & editing: all authors.