 |
 |
- Search
| Clin Exp Reprod Med > Volume 49(1); 2022 > Article |
|
Abstract
Objective
This study aimed to describe a distinct subpopulation of azoospermic patients with isolated elevation of follicle-stimulating hormone (iFSH) and poor outcomes of microdissection testicular sperm extraction (microTESE).
Methods
A retrospective analysis of microTESE outcomes was conducted among 565 patients with non-obstructive azoospermia (NOA). Testicular pathology was assessed by the dominant histological pattern and Bergmann-Kliesch score (BKS). Descriptive statistics were presented for the iFSH subgroup. Inhibin B levels, the sperm retrieval rate (SRR), and BKS were compared in iFSH patients and other NOA patients.
Results
The overall SRR was 33.3% per microTESE attempt. The median BKS was 0.6 (interquartile range, 0–2). Of all NOA patients, 132 had iFSH, and microTESE was successful only in 11 of those cases, with an SRR of 8.3%, while the total SRR in other NOA patients was 38.1% (p<0.001). iFSH had a sensitivity of 32.1% (95% confidence interval [CI], 27.4%–36.8%) and specificity of 94.1% (95% CI, 90.8–97.5%) as a predictor of negative microTESE outcomes.
Azoospermia is a highly adverse finding in the male partners of infertile couples. Although a minority of azoospermic men may benefit from surgical interventions and medical therapy, eventually leading to an emergence of sperm in the seminal fluid, most of them are naturally considered sterile and depend on sperm retrieval techniques in order to achieve biological fatherhood. However, successful surgical sperm retrieval may be guaranteed only in cases of obstructive azoospermia. In contrast, in non-obstructive azoospermia (NOA), which is characterized by spermatogenic failure, the sperm retrieval rate (SRR) tends to be modest at best. The SRR in NOA essentially depends on the surgical technique and testicular phenotype. Microdissection testicular sperm extraction (microTESE), originally proposed by Schlegel and Li [1], is the most appropriate technique for NOA. As for the testicular phenotype, there is no way of properly determining it before testicular biopsy per se, which substantially limits our understanding of the likelihood of sperm retrieval before attempting microTESE and makes patient counseling suboptimal. Even diagnostic testicular biopsy, as demonstrated by Berookhim et al. [2], has little predictive value due to the potential heterogeneity of seminiferous tubules and the random nature of this procedure.
Endocrine parameters and testicular volume are currently viewed as possible markers of microTESE success. Follicle-stimulating hormone (FSH), despite being routinely used by clinicians in decision-making along with inhibin B, seems to be quite unreliable, with a poor area under the curve (AUC) value demonstrated in a meta-analysis based on five studies [3]. Even if we consider it to be a predictor for successful sperm retrieval, it is definitely not a linear one. Indeed, a weak U-shaped relationship between FSH level and the SRR was noted in at least 1 study [4].
It would be more logical to try to integrate these clinical and biochemical parameters together in order to identify basic patterns that correspond to distinct testicular phenotypes in NOA. In other words, the likelihood for successful sperm retrieval does not and should not depend on individual endocrine parameters. Rather, using endocrine parameters as a whole, we may deduce the current intrinsic state of testicular parenchyma, and the latter effectively defines the SRR. The evaluation of clinical data, hormone levels, and testicular pathology is currently disjointed. The widely used classification, which includes hypospermatogenesis, maturation arrest, and Sertoli cell-only (SCO) syndrome, does not reflect all the complexities that underlie these phenomena.
We aimed to perform a heuristic identification of basic testicular phenotypes by comparing observed clinical patterns with pathology results and reproductive outcomes. An often overlooked clinical pattern is isolated FSH elevation (iFSH) in the presence of normal or borderline luteinizing hormone (LH) and testosterone levels. This finding is quite frequent in NOA, but it is not as widely discussed as more obvious and well-known patterns, such as hypergonadotropic hypogonadism. Therefore, we decided to begin with an attempt to describe iFSH, which seems to be the most unfavorable endocrine pattern, as pointed out by Esteves et al. [5]. Our hypothesis was that iFSH may reflect a specific testicular phenotype characterized by a negligible SRR and adverse pathological findings.
A retrospective analysis of microTESE outcomes was conducted among 565 patients with primary infertility and NOA, who were treated at our center between October 2010 and December 2017. This retrospective study was approved by the Institutional Review Board of V.I. Kulakov National Medical Research Center. The World Health Organization (WHO) definition of infertility was used [6]. As all included patients were azoospermic, male factor infertility was obvious. Prior to microTESE, all patients were interviewed regarding their duration of infertility, previous fertility treatments, chronic health conditions, family history, previous surgical treatments, medications, occupational hazards, and lifestyle risk factors. A physical examination, complete blood count, urinalysis, blood chemistry tests, endocrine profiling, ultrasonography, and electrocardiography were performed in all patients. Patients’ endocrine profile was evaluated using a Cobas e411 analyzer for immunochemistry testing (Roche Diagnostics, Basel, Switzerland). The inhibin B level was measured using an Inhibin B Gen II assay (Beckman Coulter United Kingdom, Wycombe, UK). Salvage microTESE procedures, as well as cases when any type of hormonal therapy was administered, were not included in this analysis.
Semen analysis was performed according to the WHO laboratory manual [7]. Azoospermia was confirmed when at least two semen analyses revealed an absence of sperm in the ejaculate. Patients with azoospermia caused by spermatogenic failure (i.e., NOA) were selected by testicular pathology results showing characteristic alterations of tubular structure (hypospermatogenesis, maturation arrest, SCO syndrome, or tubular atrophy).
All patients underwent microTESE, as originally described by Schlegel and Li [1]. Prior to the procedure all patients signed an informed consent form, which included a statement on the possible use of anonymized data for subsequent scientific analysis. The surgical approach involved an incision along the scrotal raphe and separation of the tunica dartos, with subsequent testis delivery. In several complex cases, when vasal or epididymal obstruction amenable to reconstruction was suspected (the precise nature of azoospermia was not yet known because there were no pathology results), lateral scrotal incisions were used instead, allowing the vas deferens to be inspected, but then, as it became obvious that the patient had NOA, regular sperm retrieval was commenced. When the seminiferous tubules were distinctly heterogeneous under optical magnification (×15–20), we performed dissection in order to find more or less intact tubules and attempted sperm retrieval, while taking one or two pieces of testicular parenchyma for a pathological examination. When the seminiferous tubules were visually judged to be of poor quality, the parenchyma appeared homogeneous, and a thorough dissection revealed no healthy tubules, we attempted random biopsies from different areas. During all steps of this procedure, an embryologist was present nearby the operating room to handle the specimens and to perform an initial search for viable sperm, guiding the ongoing surgical procedure. The tissue samples underwent standard mechanical processing, which included mincing and passing the suspension through a 24-G catheter multiple times, prior to microscopy.
In this retrospective study, we analyzed the following variables for each sperm retrieval attempt: patient’s age, serum levels of inhibin B, testosterone, LH, FSH, estradiol, sonographically measured total testicular volume (using a built-in formula: length×width×height×0.52), presence of varicocele, presence of genetic causes of testicular failure (e.g., azoospermia factor deletions, Klinefelter syndrome, and other karyotype abnormalities), history of cytotoxic chemotherapy, and previous scrotal/inguinal surgery. The definition of iFSH was based on local laboratory reference values; it was defined as a serum FSH level >12.4 mIU/mL and an LH level ≤8.6 mIU/mL. Characteristics of the study population are shown in Table 1. If any individual case lacked some of the aforementioned data, it was excluded from final analysis.
We evaluated sperm retrieval as a direct outcome of the microTESE procedure. It should be emphasized that individual sperm retrieval attempt was considered to be successful when sperm of appropriate quality were found in sufficient quantity for cryopreservation and/or immediate intracytoplasmic injection (ICSI). Cases where solitary non-viable or non-usable sperm were found during microTESE were dismissed as failed attempts. A receiver operating characteristic (ROC) curve analysis was performed for potential predictors of successful sperm retrieval.
We also evaluated the live birth rate (LBR) in the wives of patients who were included in this study. The precise embryological outcomes of ICSI such as the fertilization rate, embryo quality, biochemical pregnancy rate, and clinical pregnancy rate, were not studied, as this information was not available for a significant proportion of patients; however, this gap in the data is not critically important considering the nature of this study and the availability of final LBR data.
The testicular pathology results were stratified into hypospermatogenesis, maturation arrest, SCO, and complete tubular atrophy according to the predominant pattern as identified by a pathologist. Considering possible inconsistencies between this “dominant pattern” and the true likelihood of successful sperm retrieval, we also evaluated the percentage of tubules with different stages of spermatogenesis (no spermatogenesis, maturation arrest with presence of round spermatids, and complete spermatogenesis). In order to facilitate a statistical analysis, we also presented this data as a numerical variable based on the Bergmann-Kliesch score (BKS) [8]. SCO was defined as a complete absence of germ cells, with only Sertoli cells being present in a sample. Maturation arrest was reported if seminiferous tubules contained germ cells, but none of them had reached beyond a specific stage of spermatogenesis (such as round spermatids, spermatocytes, or spermatogonia). If the sample contained elongated spermatids, but in fewer than 75% of seminiferous tubules (BKS <8), the term “hypospermatogenesis” was applied. Finally, samples with a BKS of 8–10 were considered to demonstrate preserved normal spermatogenesis.
Descriptive statistics were presented for the iFSH subgroup. The normality of the distribution of numerical variables was checked with the Kolmogorov-Smirnov test. We compared the SRR as a primary endpoint in azoospermic iFSH patients and in other NOA patients using the chi-square test. The BKS and levels of inhibin B (thought to be more or less independent of gonadotropin signaling) were compared using the Mann-Whitney U-test. The difference in the SRR between iFSH patients and the rest of the sample was used as the primary outcome measure, and differences in BKS and inhibin B levels were secondary outcome measures. The p-values <0.05 were considered to indicate statistical significance. IBM SPSS ver. 23 (IBM Corp., Armonk, NY, USA) was used for the statistical analysis.
Sperm were successfully retrieved in 188 cases, and the overall SRR was 33.3% per microTESE attempt. ICSI with surgically retrieved spermatozoa led to 78 live births overall. The LBR was 13.8% per couple. The ROC analysis for potential predictors of microTESE success revealed that the AUC for inhibin B was 0.763 (95% confidence interval [CI], 0.718–0.808) (Figure 1). The AUC for testosterone was 0.543 (95% CI, 0.492–0.594), and the AUC for testicular volume was 0.762 (95% CI, 0.718–0.807). Inverted ROC curves for gonadotropin levels are shown in Figures 2 and 3. The AUC for FSH was 0.704 (95% CI, 0.654–0.754), while LH performed poorly, with an AUC of 0.608 (95% CI, 0.555–0.66).
SCO or severe atrophy affecting 100% of the seminiferous tubules was found in 183 cases (32.4%). Complete maturation arrest was relatively rare, and was found in nine cases (1.6%). In 63 cases (11.1%), SCO histology and maturation arrest overlapped. In the remaining 310 cases (54.9%), at least 1% of the seminiferous tubules contained elongated spermatids, which may be described as hypospermatogenesis.
Only a minority of cases (n=27, 4.8%) had a BKS of 8–9, which presumably corresponds to normal spermatogenesis at the site where the biopsy was taken (not reflecting spermatogenesis in the entire testes). No patients had a BKS of 10. In 201 cases (35.6%), the BKS was within the range of 1 to 7, which is interpreted as mixed testicular atrophy. In 123 cases (21.8%), the BKS was below 1, meaning that only 1%–9% of tubules contained elongated spermatids. Thus, the median BKS was 0.6 (interquartile range, 0–2).
Among 27 biopsy attempts with a BKS of 8–9 on pathology, 24 were successful (88.9%). The three failures are explained by the fact that not enough viable sperm usable for ICSI were found despite the presence of elongated spermatids. Since we do not perform elongated spermatid injection, these attempts were deemed unsuccessful. Eighty-five biopsy attempts (42.3%) were successful in patients with mixed testicular atrophy, while, surprisingly, 79 biopsy attempts (64.2%) were successful when the BKS was below 1. As expected, the SRR was 0% when all seminiferous tubules had SCO or complete atrophy.
Overall, 132 of all NOA patients had iFSH prior to the sperm retrieval attempt. MicroTESE was successful in only 11 cases. The SRR in this category was 8.3%. In comparison, the total SRR of other NOA patients taken together was 38.1%. The difference was statistically significant (p<0.001). The odds ratio for successful sperm retrieval in the iFSH group was 0.160 (95% CI, 0.084–0.305).
Interestingly enough, only 24 patients in the iFSH group had serum testosterone concentrations below 9 nmol/L, which reflects mostly normal Leydig cell function in this subpopulation. The median inhibin B level was 31 pg/mL and 65 pg/mL in the iFSH group and the rest of the patients, respectively (p<0.001). During microdissection, the testicular parenchyma was markedly homogeneous, with the appearance of yellow-brown “barren” tubules without any sites visually resembling areas of focal spermatogenesis (Figure 4).
Indeed, on testicular pathology, 82 samples (63.6%) had complete SCO histology or tubular atrophy, and in one case (0.8%) there was complete maturation arrest. In 36 other cases (27.3%), maturation arrest co-existed with SCO histology without any evidence of advanced stages of spermiogenesis. However, in the remaining 11 cases (17.8%), at least 1% of seminiferous tubules contained elongated spermatids and microTESE resulted in positive sperm retrieval. Among them, unexpectedly, there was one case with a BKS of 8, three cases of mixed testicular atrophy (BKS of 4, 4, and 1, respectively), and the other cases had BKS of 0.1–0.6. The median BKS was 0 and 0.6 in the iFSH group and the rest of the patients, respectively (p<0.001).
We evaluated the predictive parameters of iFSH status as a potential stand-alone predictor for negative microTESE (Table 3). It demonstrated poor sensitivity (32.1%; 95% CI, 27.4%–36.8%) coupled with excellent specificity (94.1%; 95% CI, 90.8%–97.5%). The positive predictive value for microTESE failure was 91.7% (95% CI, 86.9%–96.4%), and the negative predictive value for microTESE failure was 40.9% (95% CI, 36.2%–45.5%).
FSH is one of the main hormonal factors that regulate spermatogenesis. Its biological actions are mediated through receptors located on the surface of Sertoli cells [9]. It is well known that mutations affecting FSH or FSH receptor structure may have deleterious impacts on male fertility, although such cases are rarely observed in clinical practice [10,11]. Although the idea that iFSH-associated azoospermia has very poor SRR outcomes is not essentially new, we hypothesize that it represents the most adverse phenotype in the azoospermia landscape [5].
In research and clinical practice, the FSH level is treated as a serum marker for sperm retrieval failure, but this premise is biased. There is a weak, if any, linear association between the numerical value of serum FSH concentration and the likelihood of preserved spermatogenesis. The pooled analysis by Yang et al. [12] revealed an AUC of 0.72±0.04 in a ROC curve analysis of FSH as a sperm retrieval predictor; this AUC is moderate at best and reflects the inadequacy of FSH as a standalone marker. In a recent study by Zeadna et al. [13], FSH was likewise not found to be a valuable predictor of the SSR. In a study by Kelsey et al. [14], the FSH level had a fair AUC of 0.89 in simply distinguishing azoospermic and non-azoospermic childhood cancer survivors; however, with specificity and sensitivity of 81% and 83%, respectively, in this setting it is doubtful that the FSH level would be able to solve the significantly more delicate task of distinguishing azoospermic patients with focal spermatogenesis. Controversially, some authors have even described a positive association between FSH and the SRR [15]. We assume that this discrepancy can be resolved if we do not interpret the FSH level as a continuous variable, but rather as a flag of certain testicular phenotypes, such as the one we attempted to describe in this paper.
Interestingly, FSH levels in patients with iFSH-associated azoospermia tend to be only mildly or moderately elevated. This pattern can be explained by the involvement of a large number of factors in hypothalamic-pituitary-gonadal (HPG) regulation. Severe disruption of spermatogenesis is characterized by low levels of inhibin B, which means that this negative feedback loop is inactive, leading to uninhibited LH and FSH production. However, when Leydig cells are functioning normally, testosterone and estradiol exert proper negative feedback, which limits gonadotropin release so that LH remains borderline normal and FSH remains mildly elevated. This difference is due to the different effects of gonadotropin-releasing hormone (GnRH) on their production; in short, FSH seems to be less dependent on GnRH pulse frequency and seems to have a basal constitutive secretion [16]. Furthermore, inhibin B directly blocks activin-induced FSH secretion by pituitary gonadotropes, while its influence on LH secretion is limited [17]. This scenario is possible in cases of germ cell aplasia or, possibly, selective death of germ cells due to viral infection or another testicular insult that leaves interstitial testosterone-producing cells intact. In contrast with iFSH-associated azoospermia, in complete testicular failure with impaired spermatogenesis and sex steroid synthesis, both negative feedback loops are broken, which results in full-blown hypergonadotropic hypogonadism.
As for the difference in the SRR, germ cell aplasia or precise depopulation due to currently unrecognized factors would lead to a virtually zero chance of sperm retrieval (iFSH scenario). However, gross damage to the testicular parenchyma may actually leave some tubules relatively intact, with evidence of their continuing function being masked by the overwhelming HPG response when two major loops of negative feedback are severed (the hypergonadotropic hypogonadism scenario). In fact, it is the latter scenario in which microTESE technique works best. A study by Yu et al. [18] paradoxically demonstrated that patients with narrower seminiferous tubules and higher FSH levels had better chances of successful microTESE. Their findings may actually reflect the same phenomenon and even provide some indirect morphological proof. The relatively good predictive value of FSH for sperm retrieval in conventional TESE (cTESE), as observed by Gnessi et al. [19], could be a result of bias. Patients with hypergonadotropic hypogonadism and focal spermatogenesis benefit less from cTESE than they would benefit from microTESE, which negates the principal difference between them and patients with iFSH. This results in a more uniform SRR and similarly poor outcomes in all FSH groups. Some successful cTESE attempts in mildly hypergonadotropic patients with hypospermatogenesis would be possible, which would give the appearance of a “classic” inverse relationship between FSH and the SRR. Indeed, the aforementioned meta-analysis by Yang et al. [12] showed a reasonable AUC of 0.72 for FSH, but it included studies on both cTESE and microTESE. The same logic would apply to testicular sperm aspiration (TESA), which is essentially random, like cTESE, while being less invasive. A recent study by Liu et al. [20] revealed a negative correlation between the FSH level and the SRR for TESA, as expected; however, there was a positive correlation between the FSH level and microTESE success. In a study by Zhu et al. [21], FSH had an AUC of 0.87 for successful surgical sperm retrieval, but the majority of patients underwent simple cTESE. Nonetheless, the meta-analysis by Li et al. [3], based strictly on microTESE studies, demonstrated an abysmal AUC of 0.612. The AUC in our series (0.704) is well within the range of values observed by other researchers.
In microTESE, a U-shaped association between FSH and the SRR was described. The concept of iFSH-associated azoospermia fits this concept perfectly. In these adverse cases, FSH tends to be moderately elevated, which forms a “low SRR valley” in the U-shaped FSH-SRR curve. Ramasamy et al. [4] observed this “valley” with an SRR of 51% at FSH levels less than 15 IU/mL, while the SRR in other groups was over 60%. In another study, the worst prognosis for sperm retrieval was observed for FSH levels within the 10–15 IU/mL range, though it described a specific subpopulation of patients with SCO histology on diagnostic testicular biopsy [2]. Zhang et al. [22] also demonstrated poor microTESE outcomes with a “low SRR valley” in patients with moderate elevation of FSH, although only when the testicular volume was low. The authors [22] analyzed FSH and LH levels separately, so it is unknown whether their results could be influenced by the iFSH pattern. If the “low SRR valley” hypothesis is true, it invalidates the concept of an “FSH cut-off point” to select patients in whom microTESE is allegedly pointless. However, Chen et al. [23] reportedly found that an FSH cut-off point of 19.4 IU/L predicted the absence of testicular sperm, although their study was based only on a direct comparison of average FSH levels in patients with positive and negative testicular biopsies. The mean reported FSH level in patients with successful sperm retrieval was only 7.94±4.95 IU/L, which hints at the possible contamination of this group by obstructive and transitory azoospermia cases.
Most iFSH patients had idiopathic azoospermia, which is likely due to unrecognized mutations that are deleterious for germ cells. A recent study by Das et al. [24] is of interest in this context, as idiopathic NOA patients had significantly lower (though still out of reference range) levels of FSH than patients with a known etiology of NOA. The authors [24] did not report LH levels in their cohort, so it is not possible to speculate whether their patients had iFSH. However, considering that testosterone levels were higher in idiopathic NOA patients, this might be the case. We also had seven patients who previously underwent cytotoxic cancer treatment, which, as a side effect, possibly eradicated the germ cell epithelium. Norwegian researchers specifically described this as a possible adverse event in patients treated for malignant lymphomas, naming it “exocrine hypogonadism” [25]. The FSH elevation observed in human males with age may also be related to a gradual depletion of germ cells [26]. In theory, any man who would live indefinitely long would eventually develop iFSH-elevated azoospermia.
Our study is limited by its retrospective nature, lack of an adequate control group, and the fact that iFSH definition was based on local laboratory reference values. FSH production depends on many factors beyond feedback, including the general responsiveness of the HPG axis, gonadotrope population, GnRH pulse frequency, and basal FSH production. Laboratory reference values are not universal in a biological sense, and even while cases with a moderately elevated FSH level and a borderline elevated LH level could potentially bear the same iFSH-associated phenotype, they were not considered to have iFSH due to the reliance on reference values. Therefore, results obtained in this study should not be generalized, but could be used to inform further research.
In this paper, we made an attempt to describe isolated FSH elevation as a distinct clinical pattern in patients with azoospermia, and also frequently mentioned hypergonadotropic hypogonadism as another typical pattern in azoospermic patients, mainly as a reference point for comparisons. Other specific phenotypes of NOA may exist, and they need to be properly described. In our opinion, numerical variables that reflect endocrine parameters are easy to analyze and serve as convenient candidate markers of reproductive outcomes and nomogram material, but we need to move on to recognize non-linear associations and patterns in NOA.
Figure 1.
Receiver operating characteristic curve for inhibin B as a potential predictor of successful microdissection testicular sperm extraction.
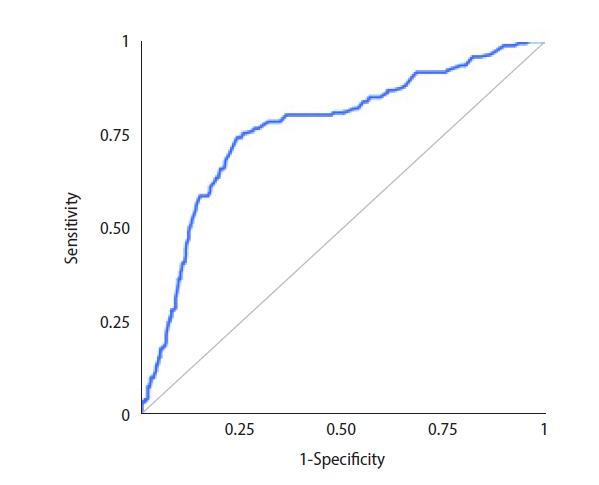
Figure 2.
Receiver operating characteristic curve for follicle-stimulating hormone as a potential predictor of successful microdissection testicular sperm extraction.
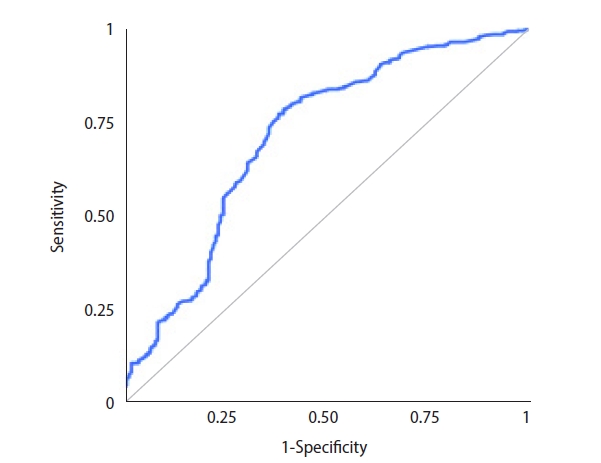
Figure 3.
Receiver operating characteristic curve for luteinizing hormone as a potential predictor of successful microdissection testicular sperm extraction.

Figure 4.
Homogenous seminiferous tubules in a patient with isolated elevation of follicle-stimulating hormone.

Table 1.
Characteristics of the study population
Table 2.
Characteristics of patients with isolated elevation of serum FSH
References
1. Schlegel PN, Li PS. Microdissection TESE: sperm retrieval in non-obstructive azoospermia. Hum Reprod Update 1998;4:439.

2. Berookhim BM, Palermo GD, Zaninovic N, Rosenwaks Z, Schlegel PN. Microdissection testicular sperm extraction in men with Sertoli cell-only testicular histology. Fertil Steril 2014;102:1282-6.



3. Li H, Chen LP, Yang J, Li MC, Chen RB, Lan RZ, et al. Predictive value of FSH, testicular volume, and histopathological findings for the sperm retrieval rate of microdissection TESE in nonobstructive azoospermia: a meta-analysis. Asian J Androl 2018;20:30-6.


4. Ramasamy R, Lin K, Gosden LV, Rosenwaks Z, Palermo GD, Schlegel PN. High serum FSH levels in men with nonobstructive azoospermia does not affect success of microdissection testicular sperm extraction. Fertil Steril 2009;92:590-3.


5. Esteves SC, Miyaoka R, Agarwal A. An update on the clinical assessment of the infertile male. [corrected]. Clinics (Sao Paulo) 2011;66:691-700.


6. Zegers-Hochschild F, Adamson GD, de Mouzon J, Ishihara O, Mansour R, Nygren K, et al. The International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary on ART terminology, 2009. Hum Reprod 2009;24:2683-7.


7. Cooper TG, Aitken J, Auger J, Baker HW, Barratt CL, Behre HM, et al. WHO laboratory manual for the examination and processing of human semen. 5th ed. Geneva: WHO Press; 2010.
8. Bergmann MN, Kliesch S. Hodenbiopsie. In: Krause W, Weidner W, editors. Andrologie. Stuttgart: Enke Verlag; 1998. p. 66-71.
9. Meduri G, Bachelot A, Cocca MP, Vasseur C, Rodien P, Kuttenn F, et al. Molecular pathology of the FSH receptor: new insights into FSH physiology. Mol Cell Endocrinol 2008;282:130-42.


10. Simoni M, Gromoll J, Nieschlag E. The follicle-stimulating hormone receptor: biochemistry, molecular biology, physiology, and pathophysiology. Endocr Rev 1997;18:739-73.


11. Themmen AP, Huhtaniemi IT. Mutations of gonadotropins and gonadotropin receptors: elucidating the physiology and pathophysiology of pituitary-gonadal function. Endocr Rev 2000;21:551-83.


12. Yang Q, Huang YP, Wang HX, Hu K, Wang YX, Huang YR, et al. Follicle-stimulating hormone as a predictor for sperm retrieval rate in patients with nonobstructive azoospermia: a systematic review and meta-analysis. Asian J Androl 2015;17:281-4.


13. Zeadna A, Khateeb N, Rokach L, Lior Y, Har-Vardi I, Harlev A, et al. Prediction of sperm extraction in non-obstructive azoospermia patients: a machine-learning perspective. Hum Reprod 2020;35:1505-14.


14. Kelsey TW, McConville L, Edgar AB, Ungurianu AI, Mitchell RT, Anderson RA, et al. Follicle stimulating hormone is an accurate predictor of azoospermia in childhood cancer survivors. PLoS One 2017;12:e0181377.



15. Amer MK, Ahmed AR, Abdel Hamid AA, GamalEl Din SF. Can spermatozoa be retrieved in non-obstructive azoospermic patients with high FSH level? A retrospective cohort study. Andrologia 2019;51:e13176.


16. Marques P, Skorupskaite K, George JT, Anderson RA. Physiology of GnRH and gonadotropin secretion [Internet]. Endotext, MDText.com; 2018 [cited 2021 Apr 29]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK279070/
17. Namwanje M, Brown CW. Activins and inhibins: roles in development, physiology, and disease. Cold Spring Harb Perspect Biol 2016;8:a021881.



18. Yu Y, Xi Q, Wang R, Zhang H, Li L, Zhu H, et al. Intraoperative assessment of tubules in predicting microdissection testicular sperm extraction outcome in men with Sertoli cell-only syndrome. J Int Med Res 2019;47:722-9.


19. Gnessi L, Scarselli F, Minasi MG, Mariani S, Lubrano C, Basciani S, et al. Testicular histopathology, semen analysis and FSH, predictive value of sperm retrieval: supportive counseling in case of reoperation after testicular sperm extraction (TESE). BMC Urol 2018;18:63.



20. Liu YP, Qi L, Zhang NN, Shi H, Su YC. Follicle-stimulating hormone may predict sperm retrieval rate and guide surgical approach in patients with non-obstructive azoospermia. Reprod Biol 2020;20:573-9.


21. Zhu ZG, Zhao ZG, Pang QY, Chen T, Zhang JM, Zhang TJ, et al. Predictive significance of serum inhibin B on testicular haploid gamete retrieval outcomes in nonobstructive azoospermic men. Asian J Androl 2019;21:137-42.


22. Zhang H, Xi Q, Zhang X, Zhang H, Jiang Y, Liu R, et al. Prediction of microdissection testicular sperm extraction outcome in men with idiopathic nonobstruction azoospermia. Medicine (Baltimore) 2020;99:e19934.



23. Chen SC, Hsieh JT, Yu HJ, Chang HC. Appropriate cut-off value for follicle-stimulating hormone in azoospermia to predict spermatogenesis. Reprod Biol Endocrinol 2010;8:108.



24. Das A, Halpern JA, Darves-Bornoz AL, Patel M, Wren J, Keeter MK, et al. Sperm retrieval success and testicular histopathology in idiopathic nonobstructive azoospermia. Asian J Androl 2020;22:555-9.



25. Kiserud CE, Fossa A, Bjoro T, Holte H, Cvancarova M, Fossa SD. Gonadal function in male patients after treatment for malignant lymphomas, with emphasis on chemotherapy. Br J Cancer 2009;100:455-63.



26. Veldhuis JD, Iranmanesh A, Demers LM, Mulligan T. Joint basal and pulsatile hypersecretory mechanisms drive the monotropic follicle-stimulating hormone (FSH) elevation in healthy older men: concurrent preservation of the orderliness of the FSH release process: a general clinical research center study. J Clin Endocrinol Metab 1999;84:3506-14.


- TOOLS







