Efficacy of methotrexate therapy in patients with tubal pregnancy and a serum human chorionic gonadotropin level above 10,000 IU/L
Article information
Abstract
Objective
To estimate the failure rate of medical treatment and to identify variables associated with treatment failure in patients with tubal pregnancy and an initial serum level of human chorionic gonadotropin (HCG) over 10,000 IU/L.
Methods
The inclusion criteria were tubal pregnancy diagnosed using ultrasonography, primary treatment of intramuscular methotrexate injection at one of the four institutions between January 2003 and December 2011, a serum HCG level within two days before treatment>10,000 IU/L, and follow-up data to determine treatment success or failure. Exclusion criteria were other primary treatments besides intramuscular methotrexate injection. The clinicopathologic data of 36 patients were collected and analyzed.
Results
Medical treatment failed and surgery was performed in 19 (53%) patients. In univariable analysis, age, parity, and size of the gestational sac were associated with treatment failure, but none of the variables were associated with treatment failure in multivariable analysis. The failure rate in the subgroup with age<33 years and size of gestational sac≥1.1 cm was significantly higher than those of the other subgroups (82% vs. 41% [mean of the other subgroups], respectively).
Conclusion
Patients with a serum HCG level>10,000 IU/L who received medical treatment had a high failure rate. Among them, patients aged<33 years and with a gestational sac≥1.1 cm had an extremely high failure rate.
Introduction
Ectopic pregnancy is a common and potentially life-threatening condition. Specifically, the prevalence of ectopic pregnancy estimated by the Centers for Disease Control and Prevention is 2% of reported pregnancies [1]. In addition, 9% of all maternal deaths are attributable to ectopic pregnancy [1].
Ectopic pregnancy can be treated medically or surgically. In a properly selected population, medical treatment is highly effective. According to a systematic review including 503 patients with medically treated ectopic pregnancy, only 20 (4%) of 503 patients experienced treatment failure [2]. However, medical treatment is not suitable for some patients. The American College of Obstetricians and Gynecologists [3] has recommended medical treatment for patients who are hemodynamically stable with no evidence of rupture. In addition, a systematic review suggested that an high initial serum level of human chorionic gonadotropin (HCG) is a relative contraindication for medical treatment because of its high failure rate [2].
The failure rate according to the initial serum HCG level was reported in a systematic review [2]. Specifically, the failure rate in patients with HCG<5,000 IU/L was <4%. However, the failure rate in patients with HCG 5,000 to 9,999 and ≥10,000 IU/L were 14% and 18%, respectively. The authors concluded that medical treatment should be used with caution in patients with a high initial serum HCG level. However, those failure rates were calculated from a small number of patients; only 22 patients with HCG≥10,000 IU/L were included in the analysis. In addition, none of the five studies included in the systematic review were prospective studies.
There are some patients who choose medical treatment despite the anticipated high failure rate. For example, women with previous unilateral salpingectomy may choose medical treatment over surgery. In addition, the 18% failure rate in patients with HCG≥10,000 IU/L might be an acceptable rate in regions where the emergency unit is easily accessible. However, to the best of our knowledge, the literature addressing the failure rate in patients with HCG>10,000 IU/L is sparse.
The objectives of this study were to estimate the failure rate of medical treatment and to identify variables associated with treatment failure in patients with tubal pregnancy and an initial serum HCG level>10,000 IU/L.
Methods
1. Patients
The inclusion criteria were 1) tubal pregnancy diagnosed using ultrasonography, 2) primary treatment of intramuscular methotrexate injection at one of the four institutions between January 2003 and December 2011, 3) a serum HCG level within two days before treatment>10,000 IU/L, and 4) follow-up data to determine treatment success or failure. Treatment success was defined as no surgery until the serum HCG level fell below 500 IU/L or until three months after intramuscular methotrexate injection. Treatment failure was defined as surgery for tubal pregnancy with any indication. Exclusion criteria were any other primary treatment besides intramuscular methotrexate injection.
After the approval of each institutional review board, the clinicopathologic data of 36 patients were collected from all four institutions. The variables collected were age, parity, gravidity, gestational age, pretreatment serum HCG level, pretreatment ultrasonography findings, regimen of methotrexate [1], lowest serum HCG level from day 4 and 10 (day 1 is the treatment day), and indication of surgery.
2. Analysis
The characteristics of the patients were identified. The failure rate was calculated by dividing the number of patients who received surgery by the total number of patients. The association of variables with treatment failure was evaluated using the Wilcoxon test for continuous variables and Fisher's exact test for categorical variables. Variables associated with treatment failure in univariable analysis were included in the model for multivariable analysis. Null hypotheses of no difference were rejected if p-values were less than 0.05. All analyses were performed using SPSS ver. 20.0 (SPSS Inc., IBM Co., Armonk, NY, USA).
Results
1. Characteristics
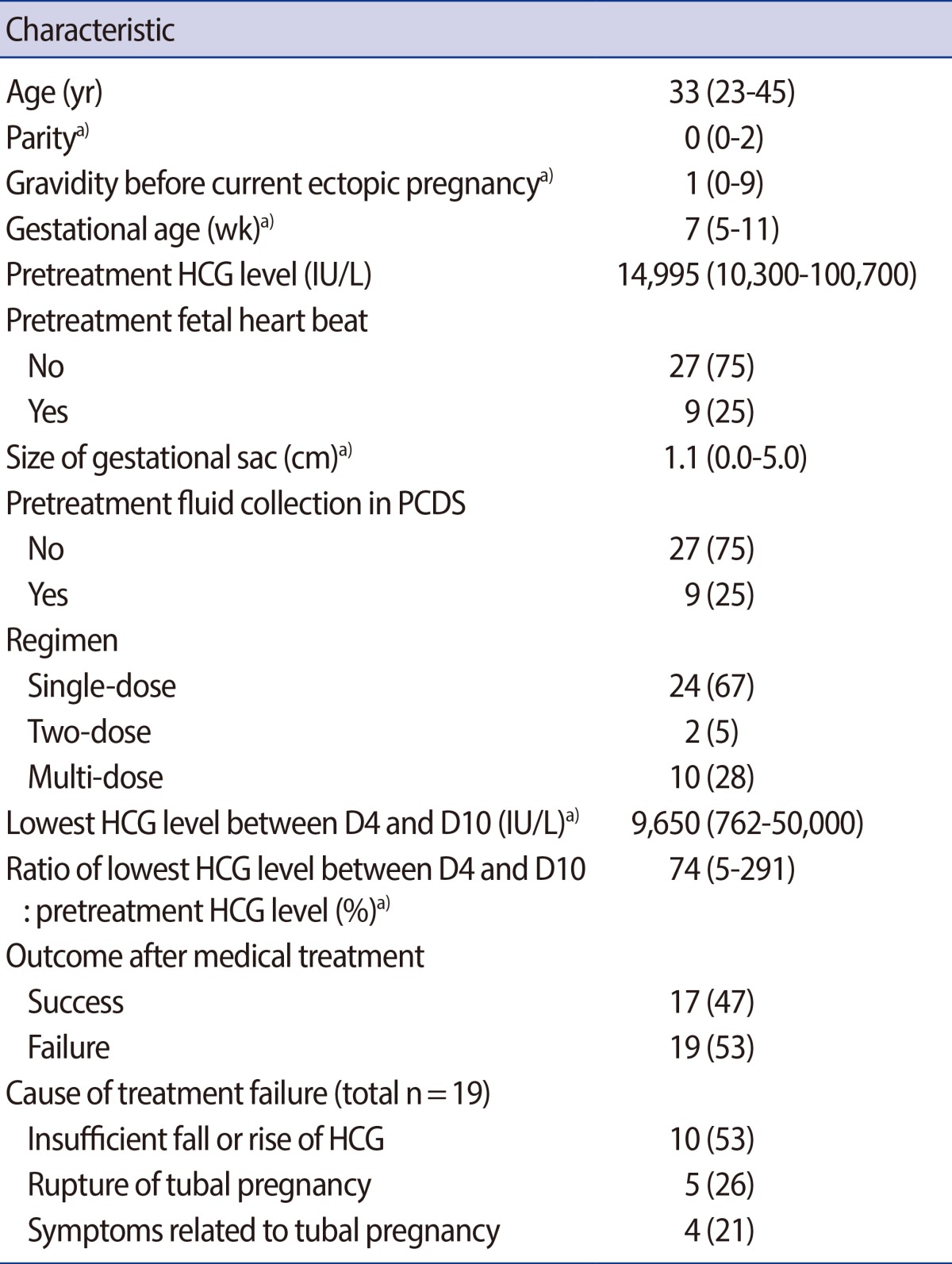
The characteristics of the 36 patients are summarized in Table 1. The median age was 33 years. The median parity and gravidity were 0 and 1, respectively. The median gestational age was 7 weeks and median pretreatment serum HCG level was 14,995 IU/L. A fetal heart beat was observed in nine (25%) patients and the median size of the gestational sac was 1.1 cm. Fluid collection in the posterior cul-de-sac was observed in nine (25%) patients. Single-dose, two-dose, and multi-dose regimens were used in 24, 2, and 10 patients, respectively. Between day 4 and 10, the serum HCG level fell to 74% of the pretreatment level and median serum HCG level was 9,650 IU/L. Surgery was performed in 19 (53%) patients. The indication of surgery was an insufficient fall or rise of HCG in 10 patients, rupture of tubal pregnancy in 5, and symptoms related to tubal pregnancy (abdominal pain) in 4.
2. Association of variables with treatment failure
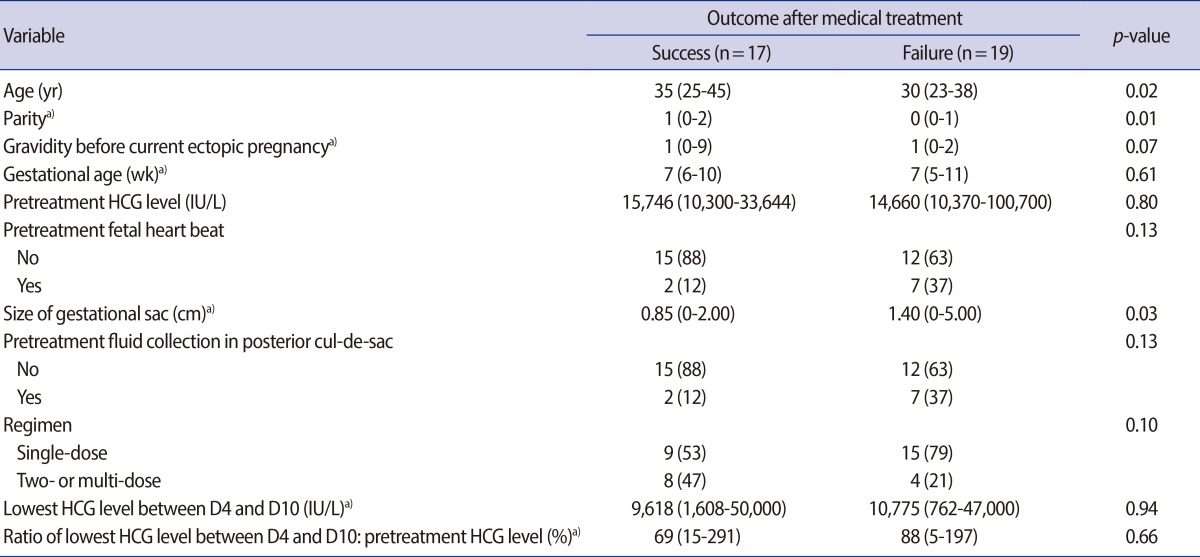
In univariable analysis, age, parity, and size of gestational sac were associated with treatment failure. Specifically, patients with younger age, low parity, and a larger gestational sac were more likely to fail treatment (Table 2). However, no variables were associated with treatment failure in multivariable analysis (data not shown).
3. Failure rate according to age and size of gestational sac
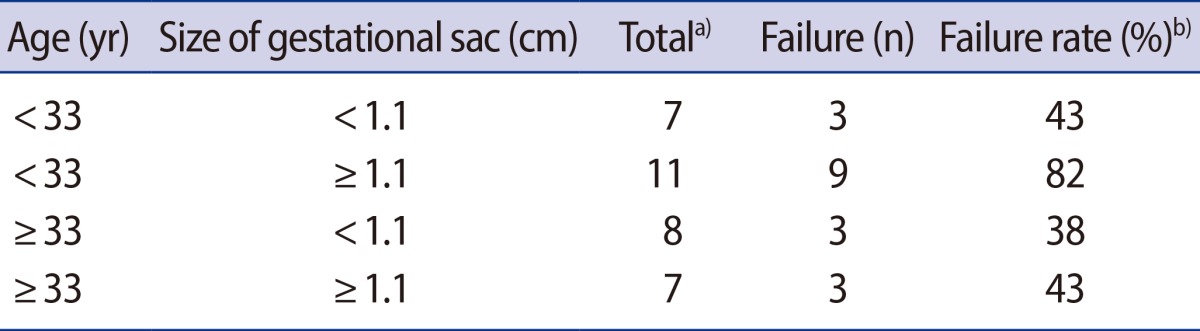
Using age and size of gestational sac, which were associated with treatment failure in univariable analysis, we divided patients into four subgroups and estimated the failure rate in each subgroup. The failure rate in the subgroup with age<33 years and size of gestational sac≥1.1 cm was significantly higher than those of the other subgroups (82% vs. 41% [mean of the other subgroups]) (Table 3).
Discussion
We found that a very high rate of treatment failure (53%) in patients with an initial serum HCG level>10,000 IU/L. The failure rate is much higher than the rate in previous studies. For example, the failure rate was 18% in a systematic review [2] and 25% in the largest, single-center study [4].
An unclear indication for surgery could be the cause for the high failure rate. In our study, the indication for surgery was insufficient fall or rise of HCG in 10 patients, rupture of tubal pregnancy in 5, and symptoms related to tubal pregnancy (abdominal pain) in 4. We conjecture that surgery might have been unnecessary in many patients with an insufficient fall or rise of HCG or symptoms related to tubal pregnancy. In one study, over half of patients who received medical treatment experienced an initial rise of serum HCG, but medical treatment was successful in 62% of patients with a rise of serum HCG [5]. Similarly, several other studies reported that a significant number of patients with an initial rise of serum HCG eventually achieved treatment success [6,7]. Symptoms related to tubal pregnancy are not reliable indicators for surgery. To understand this better, 53 patients with ectopic pregnancy requiring evaluation for pain after medical treatment were included in a study. Ultimately, only 10 of 53 patients underwent surgery. The majority of patients with pain, even with rebound tenderness or free peritoneal fluid, were able to be treated successfully without surgery [8].
The decision to perform an operation is a complex process involving medical and non-medical issues. To accurately evaluate the failure rate of medical treatment, a prospective study would be necessary.
Many factors are thought to raise the risk for treatment failure in patients with ectopic pregnancy who have received medical treatment. For example, women with a high serum HCG level are more likely to experience treatment failure [2]. Ultrasonographic evidence of fetal heart beat was associated with treatment failure [9]. Other variables such as size of the ectopic mass, peritoneal fluid, ultrasonographic evidence of a yolk sac, isthmic location of the ectopic mass, high pretreatment folic acid level, and rate of HCG rise or fall following treatment were associated with treatment failure [4,10,11,12]. In the current study, the association of risk factors with treatment failure was not verified. Only age, parity, and size of gestational sac were associated with treatment failure in univariable analysis. The discordance between previous studies and our study could be due to the different population characteristics (initial level of HCG>10,000 IU/L) or small sample size of the current study.
Although we did not find variables associated with treatment failure in multivariable analysis, we identified a subgroup with the highest failure rate. Patients with age<33 years and size of gestational sac≥1.1 cm had an extremely high failure rate (82%). In addition, three of five women with the rupture of a tubal pregnancy were included in this subgroup.
Our study has several limitations. First, our study is a small, retrospective study. Second, the regimen of methotrexate and the indication for surgery were varied. However, to our knowledge, this is the second largest, multi-institutional study regarding the treatment outcome of patients with an initial serum HCG level>10,000 IU/L.
In conclusion, patients with a serum HCG level>10,000 IU/L who received medical treatment had a high failure rate. Notably, patients with age<33 years and size of gestational sac≥1.1 cm had an extremely high failure rate. However, to accurately estimate the failure rate, a prospective study is necessary.
Acknowledgments
The authors thank the Medical Research Collaborating Center at Seoul National University Bundang Hospital for statistical analyses and appreciate the help given by Jeongha Kim and Seungmi Lee regarding data collection.
Notes
No potential conflict of interest relevant to this article was reported.