Glucocorticoid therapy in assisted reproduction
Article information
Abstract
As glucocorticoids are well-known as important regulators of stress and the immune system, their function and clinical use have elicited substantial interest in the field of reproduction. In particular, the effect of glucocorticoid therapy on endometrial receptivity during assisted reproduction, including in vitro fertilization (IVF) cycles, has led to a great deal of interest and controversy. However, previous studies have not been able to provide consistent and reliable evidence due to their small, non-controlled designs and use of different criteria. Considering the potential risk of exposure to glucocorticoids for mothers and fetuses in early pregnancy, the use of glucocorticoids in IVF cycles should be carefully evaluated, including the balance between risk and benefit. To date, there is no conclusive evidence that the use of glucocorticoids improves the pregnancy rate in IVF cycles with unselected subjects, and a further investigation should be considered with a proper study design.
Introduction
Achieving pregnancy with assisted reproductive technology can be affected by multifactorial causes. To date, numerous studies have documented ways of improving the outcomes of in vitro fertilization (IVF), the most important assisted reproductive technique clinically used to manage infertility. These efforts include optimizing IVF procedures in terms of ovarian stimulation [1], oocyte retrieval [2], fertilization [3], and the embryo culture system [4]. However, the current technique as relates to the steps from embryo transfer to implantation—the final hurdle to pregnancy—remains difficult to consider as having been optimized [5]. The uterine endometrium is the last barrier to overcome in order to make further progress in assisted reproductive technology [6].
Endometrial receptivity can be defined as the capacity for endometrial maturation, during which the trophectoderm of the blastocyst can attach to the endometrial epithelial cells and subsequently proceed to invade the endometrial stroma and vasculature [7]. It provides the embryo with the opportunity to attach, invade, and develop in the maternal uterine environment, within the exceptional 3- to 5-day period known as “the window of implantation.” Various strategies for improving endometrial receptivity have been studied, including identifying biomarkers to schedule embryo transfer [8,9], endometrial scratching [10], applying medication or materials to the uterine endometrium [11,12], and even a freeze-all strategy after IVF with controlled ovarian stimulation [13]. However, the strategy that induces optimal endometrial receptivity has not yet been confirmed clinically.
Previous studies have reported that the immune system plays a central role in endometrial receptivity, with resident immune cells modulating the decidual response, epithelial attachment of the embryo, trophoblast invasion, vascular adaptation, and immune tolerance [14-16]. Based on this hypothesis, glucocorticoids have been proposed as a way to improve the embryo implantation rate after IVF and to protect against miscarriage, when administered during embryo implantation through the early placentation phase [17,18]. However, no well-designed clinical studies have offered acceptable conclusions regarding the indications, effectiveness, and safety of adjuvant corticosteroid therapy in IVF cycles. This review aimed to evaluate previous studies on glucocorticoid therapy during IVF cycles and to elucidate weak points to address in future research.
Glucocorticoids and reproduction
Glucocorticoids are essential steroid hormones that regulate diverse cellular functions and are indispensable for maintaining normal physiology by inducing the capacity to respond appropriately to stress through the regulation of metabolic activity, behavior, and even reproduction. Glucocorticoids are synthesized and released by the adrenal cortex under the regulation of the hypothalamus-pituitary-adrenal (HPA) axis, in a pulsatile pattern showing both circadian and ultradian rhythms. Corticotropin-releasing hormone (CRH) and arginine vasopressin are secreted from the parvicellular neurons of the hypothalamus into the pituitary portal circulation, thereby stimulating adrenocorticotropic hormone (ACTH) release from the anterior pituitary gland [19]. ACTH stimulates the adrenal gland to induce steroidogenesis and the production of glucocorticoids. Increased glucocorticoid levels, in contrast, inhibit CRH expression and its secretion, as well as ACTH output by endocrine feedback loops [20]. This rhythmic regulation of glucocorticoid levels is critical for the maintenance of physiological homeostasis and adjustment to acute stress exposure by transiently inducing HPA activity. However, this regulation is not restricted to the HPA axis; instead, it also involves adjustments at the systemic level. The enzyme 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) produces cortisol by the enzymatic reduction of cortisone, and the reverse reaction is catalyzed by 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2) [21]. Additionally, the glucocorticoid receptor (GR) mediates glucocorticoid activity in a diverse manner, ranging from stimulation to suppression in a cell-specific manner [22-24]. The GR is a member of the nuclear receptor superfamily of ligand-dependent transcription factors and has a modular structure composed of three distinct functional domains [25]. Between the DNA-binding domain and the ligand-binding domain lies a flexible hinge region that provides structural flexibility for genomic interactions and contains a nuclear localization signal [26].
Increased glucocorticoid levels, resulting from either endogenous or exogenous processes, cause various types of reproductive dysfunction via effects in the hypothalamus and pituitary gland within the HPA axis [27]. Recent animal studies suggested that glucocorticoids disturb the hypothalamus-pituitary-ovary axis by direct inhibition of gonadotropin-releasing hormone (GnRH) secretion from the hypothalamus and the synthesis and the release of gonadotropins from the pituitary by suppressing kisspeptin (KISS1) [28,29] and inducing gonadotropin-inhibitory hormone [30,31].
In addition to the effects in the upper neuro-endocrine area, glucocorticoids are known to impact ovarian cyclic physiology and steroidogenesis directly by regulating the functions of granulosa cells, oocytes, cumulus cells, and luteal cells [29,32]. Increased glucocorticoid levels impair the developmental competence of oocytes by triggering apoptosis of granulosa cells [33]. Glucocorticoids differentially induce and repress steroidogenesis in the ovary according to their dose or the stage of follicular development [32,34,35]. The complexity of the relationship between glucocorticoids and ovarian function remains to be elucidated because most previous studies have conducted exposure experiments instead of investigating physiologic functional changes in the ovary.
Early exposure to glucocorticoids blocks estrogen-induced uterine growth, thereby reducing the quantitative capacity of implantation [36,37]. In the endometrium, GR is expressed in stromal, endothelial, and uterine natural killer (uNK) cells [38]. Given that glucocorticoids inhibit angiogenesis [39], reports that the endometrial expression of 11β-HSD1 and GR mRNA is upregulated at menstruation [40], and that the activity of glucocorticoids decreases in response to enhanced 11β-HSD2 levels in the endometrium of women with heavy menstrual bleeding [41], implicate the role of glucocorticoids in the uterine menstrual cycle.
Glucocorticoids and immune modulation
For endometrial receptivity to the semi-allogenic fetus and maintenance of successful pregnancy, various immune cells are recruited and tuned in the microenvironment of the endometrial compartment [42]. Previous studies have revealed the roles of the four main immune cell lineages: uNK cells, dendritic cells (DCs), macrophages, and T-cells. First, uNK cells are known to be important actors in decidual blood vessel modification during the implantation period [43], as well as in uterine arterial modification and optimal placentation for the development of offspring in early pregnancy [44,45]. Interacting with uNK cells, DCs have been reported to control the adaptive immune compartment and to drive the generation of inducible regulatory T cells to suppress inflammation and mediate immune tolerance of fetal antigens [46,47]. A previous study showed that depletion of uterine DCs resulted in aberrant decidual vascularization and placentation, leading to impaired implantation [48]. The M2 type of macrophages is required for embryo implantation, ovarian progesterone synthesis, and fetal development [49,50]. It was reported that M2 macrophages inhibit inflammation and contribute to immunosuppressive function by secreting anti-inflammatory cytokines [51,52]. In interacting with uNK cells and DCs for optimal implantation and successful pregnancy, regulatory T cells regulate vascular adaptation and placental development [53,54]. Some clinical studies suggested evidence that alterations of these immune cells were linked to infertility [55-58]. These immune cells and secreted mediators build on the cyclic immune changes that accompany hormonal fluctuations over the course of every menstrual cycle, particularly after boosting embryo attachment and trophoblast invasion.
Glucocorticoids could act as potent players in this process due to their potent anti-inflammatory and immunosuppressant actions. Some studies have revealed the effect of exogenous glucocorticoid exposure on these immune cells in the receptivity process [38,59]. uNK cell-mediated cytotoxicity is sensitive to exogenous glucocorticoids by regulation via GR [38,60], and the number of uNK cells in the mid-luteal endometrium decreases [61]. The function of DCs is reported to change in response to glucocorticoids, in terms of their phenotype, maturation, and antigen-presentation [62]. Corticosteroids impair the ability of DCs to activate T cells and shift the balance from cellular (Th1) immunity to humoral (Th2) immunity, and regulatory T cells are induced [63,64]. Corticosteroids shift the macrophage phenotype from pro-inflammatory (M1) to anti-inflammatory (M2), promote phagocytosis, inhibit major histocompatibility complex II expression, block the synthesis of cytokines, prostaglandins, and leukotrienes, and depress tumoricidal and microbicidal activity [63]. Glucocorticoids seem to increase the activity of regulatory T cells and decrease the cytotoxicity of NK cells simultaneously. However, recent studies on immune regulation have suggested that these regulatory activities could be individualized for each respective cell rather than being regulated in the manner of general suppression by glucocorticoids [65,66].
Glucocorticoids and the ovarian response
Some previous studies implied that glucocorticoids have positive effects on the ovarian response to stimulation. One study showed that dexamethasone may influence follicular development and oocyte maturation directly, via 11β-HSD1 in granulosa cells [67], or indirectly, by increasing serum growth hormone and intrafollicular IGF-1 levels [68,69]. The activity of 11β-HSD in ovarian follicular fluid has even been suggested as a predictive marker for IVF outcomes [70]. However, there have been few studies investigating whether glucocorticoid treatment enhances the ovarian response in IVF cycles, and the positive results showing an improved pregnancy rate were mainly reported in old preliminary studies [71]. A previous randomized controlled trial (RCT) showed a lower cycle cancellation rate in IVF cycles of normal responders using oral dexamethasone, but without differences in fertilization, implantation, and pregnancy rates [72]. A recent study suggested that low-dose oral dexamethasone in women with high progesterone levels in the early proliferative phase sensitized the ovary to gonadotropin stimulation, leading to the secretion of less progesterone, and the dexamethasone group showed a higher cumulative live birth rate than the control group [73]. Consequently, to date, the clinical evidence for using glucocorticoids to enhance the ovarian response is limited.
Glucocorticoids and embryo implantation
Historically, early experiences of using glucocorticoids in the IVF cycle were for women with positive autoantibodies. With or without low-dose aspirin, some studies reported that glucocorticoid therapy in the peri-embryo implantation period improved the pregnancy rate after IVF in women with positive anti-nuclear antibodies, anti-cardiolipin antibodies, anti-thyroid antibodies, or lupus anticoagulant [17,79,80]. Furthermore, a few studies suggested that glucocorticoid therapy could enhance the IVF pregnancy rate for women without positive autoantibodies [81] and even with unexplained repeated pregnancy loss [82,83]. In contrast to these positive studies, other studies reported that glucocorticoid therapy showed no additional benefit in implantation and pregnancy rates regardless of the dose [84-88]. These discrepant findings concerning the effectiveness of glucocorticoid therapy could be due to inconsistencies in the criteria for recruiting subjects and protocols, including the drugs utilized, dose, and schedule.
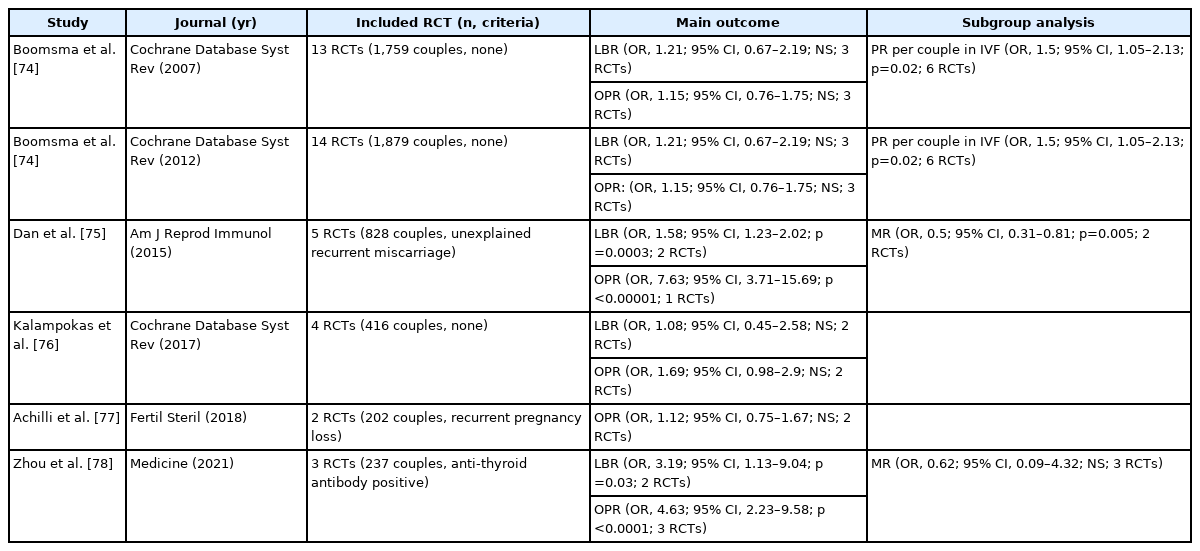
Six meta-analyses have been published regarding the effects of glucocorticoids on IVF outcomes [74-78]. The main outcomes are summarized in Table 1. In 2007, the first was published by Boomsma et al. [74], who included 13 studies and found that glucocorticoid therapy led to no significant improvement in the live birth rate (odds ratio [OR], 1.21; 95% confidence interval [CI], 0.67–2.19) or the pregnancy rate (OR, 1.15; 95% CI, 0.76–1.76). In a subgroup analysis including only fresh IVF cycles, they suggested that the pregnancy rate was significantly enhanced (OR, 1.50; 95% CI, 1.05–2.13) in the glucocorticoid treatment groups. In 2012, they reported an updated meta-analysis including 14 studies that showed similar results [74]. Dan et al. [75] reported that prednisolone therapy during IVF cycles improved pregnancy outcomes in women with idiopathic recurrent miscarriage (live birth rate: risk ratio [RR], 1.58; 95% CI, 1.23–2.02; successful pregnancy outcome: RR, 7.63; 95% CI, 3.71–15.69; miscarriage rate: RR, 0.42; 95% CI, 0.28–0.61), in five RCTs. Significant outcomes were not found in a subgroup analysis only including intracytoplasmic sperm injection (ICSI) cycles in their study. In a meta-analysis with four RCTs, Kalampokas et al. [76] reported that there was no conclusive evidence of a difference in the clinical pregnancy rate (OR, 1.69; 95% CI, 0.98–2.90) between glucocorticoid supplementation during ovarian stimulation for IVF or ICSI and the control group. A meta-analysis about the effects of various immunotherapies in IVF cycles for women with recurrent pregnancy loss revealed that prednisolone therapy also showed no significant favorable differences in the pregnancy rate (OR, 1.02; 95% CI, 0.65–1.58) in 2 RCTs [77]. Recently, a meta-analysis of the benefits of glucocorticoid treatment in infertile women with thyroid autoimmune disease during IVF cycles suggested that glucocorticoid therapy showed satisfactory effects on improving the clinical pregnancy (OR, 4.63; 95% CI, 2.23–9.58) and live birth rates (OR, 3.19; 95% CI, 1.13–9.04) in three RCTs [78]. Nonetheless, the efficacy of glucocorticoid therapy in IVF cycles remains to be elucidated, due to the limited number of included studies, as the authors pointed out in their meta-analysis.
Glucocorticoids and potential risk
Most glucocorticoids belong to category C or D according to the United States Food and Drug Administration. This indicates that animal reproduction studies without adequate and well-controlled human data or human data from investigational or marketing experience have shown adverse effects on the fetus, but the potential benefits may warrant use of the drug in pregnant women despite the potential risks. Thus, it is important to consider the benefits and risks of glucocorticoid therapy in IVF cycles, which potentially affect the early pregnancy period. Some animal studies have claimed that exposure to glucocorticoids causes fetal growth retardation, cardiovascular, metabolic, and neuroendocrine disorders, and teratogenic effects [89,90]. Some limited human studies have shown that the use of glucocorticoids spanning the first trimester might be correlated with increases in miscarriage, preterm births, gestational hypertension, and diabetes [91,92]. Although glucocorticoids do not represent a major teratogenic risk in humans, some studies have revealed a possible causal association between cleft lip and palate and the use of corticosteroids during the peri-implantation phase [91,93,94]. Unlike cases in which glucocorticoids must be used due to underlying disease, deliberate risk/benefit analyses should be carried out in cases without preexisting indications for the use of glucocorticoids in IVF cycles.
Conclusions
Glucocorticoids are important regulators of physiologic homeostasis, immune activation, and responses to inflammatory events; therefore, they also play a relevant role in reproduction. The response of endometrial and decidual immune cells required for normal implantation presents a spectrum from normal variation to the status of impaired implantation and affected placentation by corticosteroids. Previous studies have attempted to reveal the effect of glucocorticoid therapy in IVF cycles, showing some possible benefits in patients with autoimmune disease or idiopathic recurrent pregnancy loss. However, most reports were based on small, non-controlled designs with inconsistent criteria, and their conclusions cannot be interpreted as reflecting a scientific consensus. Taken together with the potential maternal and fetal risk, the use of glucocorticoids in IVF cycles should be cautious, and the balance between the risks and benefits should be considered. To elucidate solid indications and a clinical protocol to improve IVF outcomes by using glucocorticoids, further investigation should be considered in properly designed studies.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.