Anorexigenic peptide (leptin, obestatin, nesfatin-1) levels and their impact on assisted reproductive technology treatment outcomes in patients with polycystic ovary syndrome
Article information
Abstract
Objective
In this study we aimed to assess anorexigenic peptide levels in patients with or without polycystic ovary syndrome (PCOS) and their effects on assisted reproductive treatment (ART) outcomes.
Methods
A prospective case-control study was conducted in a tertiary care university-based ART clinic. Eighty-three patients were included in the study. The PCOS group included 41 patients, and the non-PCOS group included 42 controls. The 2003 Rotterdam criteria were used for PCOS patient selection. The ART indications in the non-PCOS group were tubal factor or unexplained infertility. Venous blood samples were taken on the third day of the menstrual cycle to determine the serum anorexigenic peptide levels. The enzyme-linked immunosorbent assay method was used for laboratory analyses.
Results
In the PCOS group, serum obestatin levels were significantly lower than in the control group, but serum anorexigenic peptide levels were similar in PCOS patients with or without clinical pregnancy. Ovarian hyperstimulation syndrome (OHSS) was diagnosed only in PCOS patients, and the obestatin levels of OHSS patients were significantly lower than those of other PCOS patients.
Conclusions
Baseline anorexigenic peptide levels did not affect the clinical pregnancy rate in ART cycles. Obestatin may play a role in the pathophysiology of OHSS. This possibility should be confirmed in further research.
Introduction
Polycystic ovary syndrome (PCOS) is a complex clinical and biochemical condition with signs of ovulatory dysfunction and/or hyperandrogenism during adolescence, followed by infertility due to anovulation. Approximately 5% to 10% of reproductive-age women have been diagnosed with PCOS [1]. Stein and Leventhal defined PCOS in 1935; thousands of articles on PCOS are now published annually, but the exact mechanism of the syndrome remains unknown.
Brain and peripheral tissues control reproductive function [2], and anorexigenic peptides provide communication between these two systems [3]. Leptin, obestatin, and nesfatin-1 are examples of anorexigenic peptides that may have a relationship with PCOS pathophysiology [4,5]. Leptin is an important peptide for signal transmission between the adipose tissue and the reproductive system [5]. The true effect of PCOS on serum leptin levels is unknown, as some researchers found no difference and others found higher leptin levels in PCOS patients than in healthy controls [3,6]. The granulosa and theca cells of ovarian follicles have leptin receptors, and elevated leptin levels can affect follicular growth [7]. Obestatin, a newly discovered hormone, is mainly synthesized by gastrointestinal organs [8]. Reductions in appetite and nutrient intake are its most prominent physiological effects [9]. However, the effects of obestatin on reproductive physiology are being investigated, and its exact role in PCOS pathophysiology is not known [10]. The central nervous system, especially the hypothalamus, is the primary source of nesfatin-1 [11], although insulin-synthesizing pancreatic beta-cells also synthesize nesfatin-1. As a result of interactions with pancreatic beta-cells, nesfatin-1 may play an important role in the regulation of insulin production [12]. As is the case with obestatin, firm conclusions about the effect of PCOS on serum nesfatin-1 levels are not possible because the results of studies comparing PCOS subjects with controls are scarce [4,13].
In light of the inconsistent results that have been reported on this topic, we aimed to assess serum anorexigenic peptide levels in patients with PCOS in this study and to determine their effects on assisted reproductive treatment (ART) treatment outcomes.
Methods
A case-control study was conducted among couples with primary infertility who were admitted for an ART cycle at the outpatient infertility center of our department. The study was conducted between March 2016 and December 2016. The patients were informed about the study protocol and all participants provided written informed consent. The local ethical committee of Ankara University School of Medicine approved the study protocol (No. 11-456-15). The Code of Ethics of the World Medical Association (Declaration of Helsinki) was carefully followed throughout the entire study period.
The inclusion criteria for the study were an age between 18 and 40 years, as well as a baseline follicle-stimulating hormone level between 3 and 12 IU/L. The exclusion criteria were a history of bariatric surgery, diagnosis of endometriosis with laparoscopy, a history of ovarian surgery, a chromosomal abnormality in the female partner, endocrine disorders related to female infertility, and male factor infertility.
The case group comprised patients who were diagnosed with PCOS according to the Rotterdam criteria [14] and scheduled for an ART cycle. The control group consisted of patients who were scheduled for ART either due to tubal factor infertility or unexplained infertility.
Recombinant follicle-stimulating hormone (Gonal-F, Merck-Serono, Istanbul, Turkey) was started after venous blood sampling to determine serum anorexigenic peptide levels. Gonadotropin dose adjustment was done according to age, body mass index (BMI), and the results of ovarian reserve tests. A gonadotropin-releasing hormone antagonist (Cetrotide, Merck-Serono) was generally added to the ovarian stimulation protocol (0.25 mg/day) on the sixth day of stimulation or when the leading follicle was >13 mm. When at least 3 follicles were ≥18 mm, final oocyte triggering was performed with a subcutaneous injection of 250 mg of recombinant human chorionic gonadotropin (Ovitrelle, Merck-Serono). Oocyte pick-up was performed under transvaginal ultrasound guidance 35–36 hours after the final oocyte trigger. All embryo transfers were performed with day 3 embryos. The number of transferred embryos was in accordance with national embryo transfer regulations. The luteal phase was supported with vaginal micronized progesterone (90 mg/day, Crinone 8% gel; Merck-Serono).
Clinical pregnancy was defined as the presence of an intrauterine fetal heartbeat at 6 gestational weeks. The implantation rate was calculated separately for each woman as the number of gestational sacs divided by the transferred embryos, multiplied by 100. The live birth rate was the primary outcome measure. Peripheral blood samples were obtained from the brachial vein after overnight fasting on day 3 of the menstrual cycle to determine serum anorexigenic peptide levels. Blood samples were drawn into tubes containing ethylene-diamine-tetraacetic acid (EDTA) 2Na (1 mg/mL) and aprotinin (500 U/mL). Samples were immediately centrifuged at 3000 ×g for 10 minutes at 4°C and then stored at –80°C until the day when enzyme-linked immunosorbent assay (ELISA) was performed. Serum leptin, obestatin, and nesfatin-1 analyses were done with commercial ELISA kits (DIAsource Leptin ELISA Kit [sensitivity, 0.1 ng/mL; intra-assay coefficient of variability [CV], <13.3%; inter-assay CV, <10.2%]; KAP2281, Belgium; SunRed Biotechnology human obestatin ELISA kit [sensitivity, 0.1 pg/mL; intra-assay CV, <10%; inter-assay CV, <15%], 201-12-0971, China; and SunRed Biotechnology Human Nestatin-1 ELISA kit [sensitivity, 0.113 mmol/L; intra-assay CV, <10%; inter-assay CV, <12%], 201-12-4341, China).
SPSS ver. 15.0 (SPSS Inc., Chicago, IL, USA) was used for data analysis. The Kolmogorov-Smirnov test was performed to test the normality of the distribution of the variables. According to distribution of each variable, either the Student t-test or the Mann-Whitney U-test was calculated. Categorical variables were compared using the chi-square test. A p-value of <0.05 was considered to indicate statistical significance.
Results
In the final analysis, 83 patients were eligible for the study. The study group included 41 patients with PCOS and the control group included 42 patients with either tubal factor infertility or unexplained infertility. The groups were similar in terms of baseline and demographic parameters except for baseline luteinizing hormone (LH) levels (Table 1). The features of the ovarian stimulation cycles are presented in Table 1. The clinical pregnancy rate per embryo transfer was comparable between the PCOS (35.1%) and control (35.7%) groups.
Table 1 also presents the serum anorexigenic peptide levels of both groups. The obestatin concentration was found to be significantly lower in the PCOS group (169.02 pg/mL; range, 15.42–358.98 pg/mL) than in the control group (224.51 pg/mL; range, 22.20–437.79 pg/mL) (p=0.04). The median serum leptin concentrations in the PCOS and control groups were 26.15 ng/mL (range, 4.60–88.50 ng/mL) and 27.20 ng/mL (range, 1.90–122.90 ng/mL), respectively (p=0.530). The nesfatin-1 concentrations in the PCOS and control groups were 100.47 mmol/L (range, 13.70–411.51 mmol/L) and 144.35 mmol/L (range, 26.99–450.82 mmol/L), respectively (p=0.096).
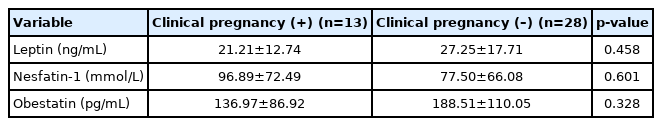
Additionally, anorexigenic peptide levels were similar in PCOS patients with and without clinical pregnancy (Table 2). The mean levels of leptin, nesfatin-1, and obestatin in patients with clinical pregnancy were 21.21±12.74 ng/mL, 96.89±72.49 mmol/L, and 136.97±86.92 pg/mL, respectively. The mean levels of leptin, nesfatin-1, and obestatin in patients without clinical pregnancy were 27.25±17.71 ng/mL, 77.50±66.08 mmol/L, and 188.51±110.05 pg/mL, respectively.
In the PCOS group, 4 embryo transfers were postponed due to the development of early ovarian hyperstimulation syndrome (OHSS). The mean obestatin level was significantly lower in patients who had both PCOS and OHSS than in patients with only PCOS (93.68±49.33 pg/mL vs. 169.97±98.33 pg/mL, p=0.04). However, the limited number of patients with OHSS limited the statistical power. We also compared the baseline and ovarian stimulation characteristics of these patients with OHSS-free PCOS patients, as shown in Table 3, and BMI was the only factor that showed a significant difference between these groups (21.7±1.2 kg/m2 vs. 26.4±5.5 kg/m2, p=0.005).
Discussion
In this study, we aimed to assess the levels of anorexigenic peptides in patients with PCOS and their effects on ART outcomes. In PCOS patients, only obestatin levels were found to be significantly lower than in controls. Baseline anorexigenic peptide levels did not show any effect on ART outcomes in our study. The most interesting finding of the study may have been that, in the PCOS group, four patients were diagnosed with early OHSS, and their baseline serum obestatin levels were significantly lower than the rest of the PCOS group.
The hormones secreted by the gastrointestinal system and the adipose tissue play important roles in the management of the reproductive axis via autocrine, paracrine, and endocrine effects. Recently, Comninos et al. [2] reviewed the effects of seven gastrointestinal system-derived hormones and five adipose tissue-derived hormones on the reproductive system. According to them, these hormones can be used exogenously to solve reproductive problems.
Leptin is the most investigated anorexigenic peptide in the literature. In our study, no significant difference in serum leptin levels was found between patients with PCOS and the control group. This finding is also supported by other studies [3,15]. The effects of leptin on ART outcomes are unclear. High leptin levels can increase the oocyte maturation and fertilization rates, but can also affect the pregnancy rate negatively. High leptin levels can cause intra-follicular hypoxia due to the suppression of steroidogenesis [16-18]. In support of the latter findings, women with low leptin levels have been reported to have better ART outcomes [19,20]. In our study, although we found lower leptin levels in women who achieved clinical pregnancy, the difference was statistically insignificant between groups.
Vascular endothelial growth factor (VEGF) is an important mediator in the pathogenesis of OHSS. Leptin levels showed positive correlations with follicular-fluid VEGF levels in previous studies, but 2 studies did not show any difference in baseline serum leptin levels between patients who did and did not develop OHSS [21,22]. Our findings are also similar to those. In the PCOS group, four patients were diagnosed with early OHSS. However, their baseline serum leptin levels were similar to those of the rest of the PCOS group
In animal studies, insulin-secreting beta-cells and cells secreting pro-nesfatin-1 (the precursor of nesfatin-1) were found in the same location in the pancreas [12]. Hyperinsulinemia is a common clinical finding in PCOS [13], and hyperinsulinemia can impair beta-cell function. As their location in the pancreas is the same, nesfatin-1 secretion might also be affected by hyperinsulinemia. This theory could explain the low nesfatin-1 levels in PCOS patients [23,24], which was also observed in our study. Considering the effects of nesfatin-1 on gonadotropin synthesis in the pituitary gland, a decrease in central nesfatin-1 levels may also increase serum LH levels in women with PCOS [25].
In a previous study, researchers evaluated the effect of nesfatin-1 levels on ART outcomes. No relationships were found between the fertilization rate, pregnancy rate, and nesfatin-1 levels. However, patients with higher intrafollicular fluid nesfatin-1 levels were found to have a higher number of oocytes and good-quality embryos available for cryopreservation [26]. In our study, we did not find a relationship between serum nesfatin-1 levels and the number of mature oocytes retrieved. However, we found higher serum nesfatin-1 levels in patients who achieved clinical pregnancy, but the difference was statistically insignificant. Larger studies, particularly focusing on histologic and embryologic follow-up, may clarify the possible effects of nesfatin-1 on ART outcomes.
Ghrelin, which was first discovered in 1999, affects luteal cell function by reducing progesterone synthesis and shifting the balance between luteotropic and luteolytic factors [27]. Obestatin, which has the same origin as ghrelin, antagonizes the effects of ghrelin [8]. Obestatin can have effects on ovarian remodeling. The first study evaluating the effects of obestatin on the reproductive system was reported in 2008. In that study, granulosa cells were exposed to obestatin, and levels of proliferation markers (cyclin B1, MAP kinase) and apoptotic peptides (BAX, caspase 3) increased in granulosa cells after obestatin exposure [28]. Low obestatin levels may play a role in the development of the ultrasonographic findings of PCOS by negatively affecting ovarian morphology and the ovarian microenvironment. In our study, patients with PCOS had significantly lower serum levels of obestatin than the control group.
OHSS is a dreaded possibility for clinicians dealing with ART, since it can cause life-threatening complications. In the PCOS group, basal obestatin levels in patients who developed OHSS were significantly lower than in those who did not. Romani et al. [29] reported that the exposure of luteal cells to obestatin reduced VEGF release, which is responsible for capillary permeability during OHSS. Obestatin may act as a protective agent against OHSS. Future studies with larger cohorts are urgently needed to clarify whether obestatin has a protective effect against OHSS.
The most important strength of our study was the systematic exploration of individual anorexigenic peptide parameters. In addition, to the best of our knowledge, this is the first study to show a significant association between decreased serum obestatin levels and OHSS. However, only four patients developed OHSS, which makes the statistical significance of the difference between groups questionable. Further studies are needed to clarify our findings.
The main limitation of this study is the lack of measurements of anorexigenic peptide levels in the follicular fluid and the fact that correlations between serum and follicular fluid levels were not determined. We measured serum anorexigenic peptide levels before commencing exogenous gonadotropins. These medications can alter serum anorexigenic peptide levels. If serum anorexigenic peptide level measurements were also done during the ovarian stimulation period, the effects of serum anorexigenic peptide levels could have been evaluated more precisely. This is another limitation of our study.
In conclusion, according to the results of our study, serum obestatin levels are significantly lower in patients with PCOS. However, neither obestatin nor the other anorexigenic peptides showed significant effects on ART outcomes. Furthermore, obestatin levels were significantly lower in PCOS patients who developed OHSS, suggesting a possible prophylactic effect of normal obestatin levels against OHSS.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Author contributions
Conceptualization: BV, BÖ. Data curation: BV, YEŞ, BİE, MS, BB, CA, RA. Formal analysis: BV, BÖ. Funding acquisition: BV, BÖ. Methodology: BV, YEŞ, BÖ, BİE. Project administration: BV, BÖ, MS, BB, CA, RA. Visualization: BV, YEŞ. Writing-original draft: BV, YEŞ. Writing–review & editing: BÖ, MS, BB, CA, RA.